Article Text
Statistics from Altmetric.com
CASE PRESENTATION
A 72-year-old woman was referred with a unilateral ovarian mass measuring 7.7×6.5 cm, pelvic pain, and elevated tumor marker (CA125: 270 U/mL). The patient had type II diabetes mellitus, hypertension, glaucoma, and a history of an ischemic cerebrovascular occlusion. She was receiving multiple drugs (acetylsalicylic acid 100 mg, fosinopril 10 mg, atorvastatin 10 mg). After pre-operative work-up including abdominal MRI showing complex ovarian mass, normal chest X-ray examination, and electrocardiography, a debulking surgery was planned with a pre-operative diagnosis of tubo-ovarian abscess or ovarian malignancy. In our clinic we triage patients with advanced ovarian cancer to either primary debulking surgery or neoadjuvant chemotherapy according to pre-operative radiologic findings, serum CA125 levels, and physical examination. Patients with CA125 levels >500 IU/mL, moderate–severe ascites, and extensive upper abdominal disease are recommended for neoadjuvant chemotherapy. As pre-operative work-up revealed low to moderate tumor burden, we proceeded directly with laparotomy.
Intra-operative evaluation revealed 500 mL of serous ascites, disseminated malignant ovarian disease with upper and lower abdominal involvement. Intra-operative frozen analysis revealed a malignant epithelial tumor of the ovary. Total modified radical abdominal hysterectomy, bilateral salpingo-oopherectomy, pelvic peritonectomy, total omentectomy, partial cystectomy, multiple mesenteric peritoneal resections were performed with no residual macroscopic disease. As this case was seen before the publication of the LIONS trial, we also performed systematic lymphadenectomy. Total operation time was 380 min, and intra-operatively, 2 units of fresh frozen plasma and 2 units of red blood cells were administered. She was transferred to the intensive care unit after surgery and transferred to routine post-operative care service after 24 hours.
On post-operative day 3, the patient developed visual hallucination with confusion. Brain MRI was normal and laboratory tests, including electrolytes, were normal. This resolved spontaneously in 24 hours and the patient was followed up between day 3 and 8 without any problems with ambulation and was receiving a regular diet. On post-operative day 8, the patient suddenly developed abdominal and flank pain due to distension. Abdominal examination revealed distension with palpation. An ultrasound disclosed a large amount of free fluid in the pelvis. On post-operative day 9, fluid accumulation increased and anasarca with bilateral grade 3 lower extremity pitting edema developed, which restricted mobilization. Physical examination did not reveal any skin flushing, urticaria, focal angioedema, stridor, or wheezing. Blood pressure was 106/62 mm Hg, her pulse was regular and ranged between 104 and 124 beats per minute. The patient was afebrile.
Dr Ureyen and Dr Iltar: what would be your consideration for further evaluation and your differential diagnosis?
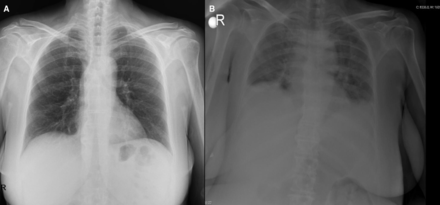
Chest X-ray examination, ultrasound examination, and abdominal CT revealed evidence of bilateral pleural effusion, free fluid in the pelvis and upper abdomen, and subcutaneous edema (Figures 1 and 2). Laboratory analysis showed hypoalbuminemia (2.3 to 1.6 g/dL) and thrombocytosis (678 to 1151×103/mm3). Hemoglobin and hematocrit levels increased from 9.4% and 27.7% to 12.1% and 36.5%, respectively. White blood cell counts also increased from 11.7 to 13.1×103/mm3. Blood creatinine level was elevated to 2.35 mg/dL (1.4 mg/dL was the baseline value for this patient). On spot urinalysis, there was no proteinuria, ruling out nephrotic syndrome, which is the main cause of hypoalbuminemia and edema. Given the daily progressive increase of fluid leakage to third spaces, biochemical and cytological analysis of free fluid was performed. Biochemical analysis revealed that serum-ascites albumin gradient was 1.5, and glucose (161 mg/dL), lipase (27 U/L), amylase (44 U/L), lactate dehydrogenase (163 U/L), triglyceride (23 mg/dL) levels were all normal. Complete microbiological analysis of blood, urine, and peritoneal fluid were performed to exclude sepsis, and these were all sterile. Hepatobiliary ultrasound examination was done to exclude portal venous thrombosis, and it was normal. Acute cardiac pathology was also excluded with electrocardiography and transthoracic echocardiography. Medical history, physical examination, absence of specific symptoms, and negative serum autoantibodies (ANA, PCNA, anti-dsDNA, SSA, SSB, scl-70, Jo-1) also excluded any possible disease of rheumatological origin.
(A) Pre-operative normal chest X-ray image. (B) Pulmonary edema with bilateral pleural effusion during capillary leakage attack.
(.A) Free fluid in the abdomen and subcutaneous edema on the sagittal plane. (B) Axial planes of the abdomen with CT. (C) Free fluid in the pelvis on ultrasound.
Dr Salim and Dr Kurtoglu: what should be the recommended approach for this patient?
Conservative treatment including fluid resuscitation with colloids and crystalloids to counteract intravascular volume depletion would be the optimal therapy. Close monitoring of fluid resuscitation is critical. While maintaining organ perfusion and avoiding metabolic acidosis, excessive fluid administration should be avoided in order to prevent compartment-related syndromes. Fluid input and output of the patient should be followed up hourly and furosemide used as diuretic therapy to prevent volume overload.
All clinical and laboratory parameters recovered, with conservative treatment, on post-operative day 20. The patient was discharged on post-operative day 26, and the first cycle of paclitaxel and carboplatin chemotherapy was administered 4 days later. Following the first cycle of chemotherapy, the patient was admitted with severe dysuria, and urine microbiological analysis showed urinary tract infection with Escherichia coli. During inpatient parenteral antibiotic therapy, she once again developed anasarca, ascites, and pleural effusion. The laboratory work-up revealed hemoconcentration (hemoglobin 17.1 g/dL, hematocrit 50.8%) and paradoxical hypoproteinemia (albumin 1.6 g/dL). With the provisional diagnosis of systemic capillary leakage syndrome, blood and urine immune fixation electrophoresis and bone morrow biopsy were performed. Monoclonal IgG kappa gammopathy and 6% lambda monoclonality in plasma cells were detected in blood immune fixation electrophoresis and bone morrow biopsy. Serum IgG level was 1260 mg/dL (normal 700–1600 mg/dL). Serum free kappa light chain and serum free lambda light chain levels increased to 74.3 mg/dL (normal 6.7–22.4 mg/dL) and 50.2 mg/dL (normal 8.3–27 mg/dL), respectively. After consultation with the hematology unit, the patient was diagnosed with systemic capillary leakage syndrome.
Due to risk of rapid progression to hypovolemic shock, the patient was transferred to the intensive care unit and 1 g/kg intravenous immunoglobulin therapy with colloid and crystalloid infusion was given for 4 days. After the third day of immunoglobulin therapy, a clinical and biochemical response (resolution of generalized edema and an increase in urine output, normalized laboratory parameters) was observed. The patient was discharged 20 days after the second attack with theophylline 200 mg two times daily and terbutaline 5 mg four times daily prophylaxis. She completed six cycles of chemotherapy 7 months after surgery under this prophylaxis regimen without any recurrence of systemic capillary leakage syndrome. The patient did not experience any systemic capillary leakage attack and there was no sign of any recurrence on her last surveillance evaluation (37 months after surgery).
Dr Dogan: please describe systemic capillary leak syndrome?
Systemic capillary leakage syndrome is a rare disorder with unexplained recurrent episodes of increased capillary permeability causing a shift of fluid and protein from the intravascular area to interstitial space. Classic diagnostic triad includes the ‘3 hour’s’ (1) hypotension (systolic blood pressure <90 mm Hg); (2) hemoconcentration (hematocrit >43%–45% in women); and (3) hypoalbuminemia (<3.0 g/dL). 1 This syndrome may be idiopathic or secondary to a triggering factor. This phenomenon was first described by Clarkson in 1960, since then, over 250 cases have been reported. 1–3 Recently, idiopathic systemic capillary leakage syndrome was classified under the paroxysmal permeability disorders, which all share some common pathophysiological mechanism caused by periodic dysfunction of endothelial permeability.4
Secondary systemic capillary leakage syndrome may be triggered by upper respiratory tract infections, sepsis, multiorgan failure, trauma, surgery, drugs, menstruation, or malignancy. 5–7 Monoclonal gammapathy of undetermined significance, genetic predisposition, and aforementioned triggering factors can be listed as risks for systemic capillary leakage syndrome. Endothelial damage is the proposed triggering mechanism but the exact cause of increased susceptibility to endothelial damage is not known. 5 Several inflammatory cytokines (TNFα, CCL2, CXCL10) and mediators of vascular permeability (VEGF, Angpt‐2) were reported to be elevated during acute episodes compared with convalescent intervals. Also activated and/or degranulated neutrophils were shown to be the contributors of acute attacks. 8 However, specific etiological factors inducing generalized systemic vascular barrier breakdown are not yet completely understood.
There are two reports of systemic capillary leakage syndrome in relation to cancer surgery. A 41 year-old woman with the diagnosis of distal bile duct cancer developed sudden and rapidly progressive severe systemic capillary leakage syndrome under general anesthesia during pancreaticoduodenectomy. The patient survived following the initiation of massive fluid infusion and cardiopulmonary resuscitation, but she developed ischemic brain injury in the recovery period. 9 In another report, a 57-year-old woman without any past medical history was diagnosed with systemic capillary leakage syndrome on post-operative day 15 after abdominoperineal resection for colorectal cancer. The patient was successfully managed with aggressive fluid administration and hemodiafiltration. The patient was discharged 10 months after the operation, and she was well without any recurrence 2 years after the surgery. 6
Surgical trauma and peri-operative pain may trigger a series of events that cause cytokine release. Moreover, hypoxemia and tissue hypoxia could decrease nitric oxide release and subsequently, could lead to endothelial dysfunction. 10 After oncologic surgery, peri-operative endothelial dysfunction was clearly documented, measured using the reactive hyperemia index, digital pulse tonometry, and biomarkers of the nitric oxide pathway. In several studies all of these measures were reduced in the early post-operative period. Such changes were reported even after minor interventions. Ovarian cancer surgery is among the most extensive and radical surgeries, including multivisceral resections and other tumorous debulking procedures. In our opinion, in our case, systemic capillary leakage syndrome could have been triggered by tissue trauma and subsequent reactions caused by extensive debulking surgery with the underlying gammopathy.
Endothelial glycocalix membrane damage is another newly described theory in systemic capillary leakage syndrome. 11 The glycocalyx plays an important role in vascular homeostasis and regulating vascular permeability. Primary injury of endothelial glycocalix can be promoted by enzymes released from damaged tissue, leukocytes (matrix metalloproteinase, hyaluronidase, heparanase), and hypoperfusion. 12 Some authors emphasized the importance of damage control surgery in order to prevent secondary causes of endothelial glycocalix damage (oxidative stress, fluid therapy, decreased oncotic pressure) in severely injured patients with trauma. 13 Damage control surgery can be defined as the prevention from the ‘lethal triad’ metabolic acidosis, hypothermia, coagulopathy during surgery. We propose that damage control surgery may be adapted to cancer surgery to decrease secondary endothelial glycocalix injury and subsequent complications.
Secondary systemic capillary leakage syndrome is a diagnosis of exclusion. Other causes of abrupt onset ascites, pleural effusion, and edema, such as sepsis, congestive heart failure, nephrotic syndrome, anaphylaxis, portal system thrombosis, should all be excluded. In our case we considered all causes of distributive, cardiogenic, hypovolemic, and obstructive shock, and all these possible causes were excluded. As the patient experienced the first attack in the immediate post-operative period before the onset of chemotherapy, it is unlikely that onset was triggered by cytotoxic agents.
Systemic capillary leakage syndrome has three phases: the prodromal, leakage, and refill phases. Symptoms such as fatigue, malaise, and flu-like disease were all described in prodromal phase, but there is no phase-specific intervention at this stage due to the vague nature of symptoms. Treatment varies on the exact phase of the disease; expanding the intravascular volume is the main goal at the leakage phase. On the contrary, hemodynamic control of excess intravascular fluid with diuretics is the standard treatment of the refill phase. 14 Empirical aggressive fluid resuscitation in the first two phases could lead to massive third spacing and progress into compartment syndrome, which may require fasciotomies, rhabdomyolysis, renal failure, and pulmonary edema. 1 Steroids have a potential role in prevention of cytokine-induced endothelial damage on disease progression. 15 Intravenous immunoglobulin has also been reported to be effective both for suppression of attacks and prevention of new episodes. 1 7 For prevention of recurrences, theophylline and β2 agonists have prophylactic effects by immunomodulation effects similar to intravenous immunoglobulin therapy. 14 Some authors have suggested use of intravenous immunoglobulin as the first-line prevention therapy. 16 In their study, multivariate analysis showed that only intravenous immunoglobulin and terbutaline therapy were substantially linked with survival. Monthly administration and no need for serum level titrations as in theophylline are the advantages of intravenous immunoglobulin prophylaxis over other protocols. However, intravenous immunoglobulin has a wide range of adverse effects, including anaphylaxis, thromboembolic events, hemolytic anemia and requires hospitalization to administer. Other drugs can be used in cases of intravenous immunoglobulin therapy failure or adverse reactions.
Dr Dogan: closing summary
Systemic capillary leakage syndrome is a life-threatening condition characterized by recurrent attacks of increased systemic vascular permeability. Exclusion of other related conditions with hyperpermeability (such as sepsis) is crucial. Optimal management and pathophysiology of systemic capillary leakage syndrome is still unclear due to the limited number of cases. Until the entire pathophysiological process is completely described, immunomodulating therapies remain the only option. In cases of unexplained refractory ascites, edema, and relevant laboratory findings following major surgeries, systemic capillary leakage syndrome should be considered.
Ethics statements
Patient consent for publication
Footnotes
Contributors SD: presenter; DK-S: medical oncologist, discussant; IU: gynecologic oncologist, discussant; UI, EK: hematologists, discussants.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Commissioned; internally peer reviewed.