Article Text
Abstract
Objectives The aim of this retrospective multicenter study was to investigate the extent, feasibility, and outcomes of minimally invasive surgery at the time of interval debulking surgery in different gynecological cancer centers.
Methods/Materials In December 2016, 20 gynecological cancer centers were contacted by e-mail, to participate in the INTERNATIONAL MISSION study. Seven centers confirmed and five were included, with a total of 127 patients diagnosed with advanced epithelial ovarian cancer after neoadjuvant chemotherapy and minimally invasive interval surgery. Only women with a minimum follow-up time of 6 months from interval surgery or any cancer-related event before 6 months were included in the survival analysis. Baseline characteristics, chemotherapy, and operative data were evaluated. Survival analysis was evaluated using the Kaplan–Meier method.
Results All patients had optimal cytoreduction at the time of interval surgery: among them, 122 (96.1%) patients had no residual tumor. Median operative time was 225 min (range 60 – 600) and median estimated blood loss was 100 mL (range 70 – 1320). Median time to discharge was 2 days (1–33) and estimated median time to start chemotherapy was 20 days (range 15 – 60). Six (4.7%) patients experienced intraoperative complications, with one patient experiencing two serious complications (bowel and bladder injury at the same time). There were six (4.7%) patients with postoperative short-term complications: among them, three patients had severe complications. The conversion rate to laparotomy was 3.9 %. Median follow-up time was 37 months (range 7 – 86): 74 of 127 patients recurred (58.3%) and 31 (24.4%) patients died from disease. Median progression-free survival was 23 months and survival at 5 years was 52 % (95% CI: 35 to 67).
Conclusions Minimally invasive surgery may be considered for the management of patients with advanced ovarian cancer who have undergone neoadjuvant chemotherapy, when surgery is limited to low-complexity standard cytoreductive procedures.
- MIS
- laparoscopy
- robotics
- IDS
- NACT
- ovarian cancer
- personalized medicine
Statistics from Altmetric.com
HIGHLIGHTS
Minimally invasive surgery was associated with a 96% rate of R0 resection at interval surgery.
The rate of intraoperative complications when performing minimally invasive interval surgery was 4.7%.
Minimally invasive interval surgery may be considered in the setting of complete or partial response after neoadjuvant chemotherapy.
Introduction
Ovarian carcinoma is the second most common gynecologic malignancy and the most common cause of death among women with gynecologic cancers.1 Women with advanced epithelial ovarian cancer have an expected long-term survival of approximately 20%.2 Standard treatment in advanced ovarian cancer is primary cytoreductive surgery followed by platinum-taxane chemotherapy,3 but over the past few decades, the use of interval surgery after neoadjuvant chemotherapy in patients with unresectable disease (International Federation of Gynecology and Obstetrics stage IIIC/ IV) or in patients with poor physical conditions, has been proposed to increase the rate of optimal debulking and reduce the number of complications, without affecting prognosis.4–7
Complete cytoreduction without macroscopic residual tumor, both as primary or interval surgery after neoadjuvant therapy is the aim of surgical management in patients with advanced ovarian cancer. In the past 5 years, technological advances, together with promising published results, has pushed the scientific community to make efforts to identify further innovative and personalized surgical treatments.8 9 However, a critical appraisal is needed regarding the surgical approach after neoadjuvant therapy. In fact, a variety of procedures are currently proposed at this time, ranging from standard cytoreductive procedures to the removal of all previously infiltrated tissues, including lymph nodes.10 Moreover, approaches may differ, with preliminary results showing minimally invasive surgery to be as effective as laparotomy at the time of interval surgery in patients with adequate response to chemotherapy.11 12
In particular, the use of 'interval' minimally invasive surgery in the specific setting of advanced ovarian cancer patients after neoadjuvant therapy is still very sporadic and has not been completely examined. Thus, the aim of this retrospective multicenter study was to investigate the extent, feasibility, and outcome of minimally invasive surgery at the time of interval surgery in different gynecological cancer centers.
Methods
In December 2016, 20 gynecological cancer centers were asked to participate in a retrospective multicenter observational study, named the INTERNATIONAL MISSION study. The centers were selected based on their experience in minimally invasive surgery, ovarian cancer treatment, and data management as assessed by their scientific publications production. The Institutional Review Board of each participating institution approved the study, and all patients signed a written informed consent to collect their data prospectively and to analyze retrospectively.
Patients with advanced epithelial ovarian cancer treated with a minimally invasive surgery after neoadjuvant chemotherapy, either robotically or laparoscopically, were eligible for the study. Inclusion criteria were histologically proven advanced epithelial ovarian cancer (International Federation of Gynecology and Obstetrics stage III–IV); unresectable disease at primary surgery confirmed either by laparoscopy, laparotomy, or combined CT scan evaluation and cytology; neoadjuvant chemotherapy (any number of cycles); and interval surgery within 45 days after completion of chemotherapy. No specific exclusion criteria were adopted and each center could freely choose the proper medical and surgical treatment for each patient. All patients not receiving standard intraperitoneal cytoreduction and with follow-up time <6 months from interval surgery were excluded from the study. Standard cytoreduction was defined as all surgical procedures (hysterectomy, salpingo-oophorectomy, omentectomy, peritoneal biopsies) that are routinely performed at the time of interval surgery independent of the presence of tumor infiltration. Cancer centers with <7 patients were also excluded. Medical records were reviewed for baseline characteristics including age, International Federation of Gynecology and Obstetrics stage, body mass index, American Society of Anesthesiologists score, pathologic characteristic and chemotherapy details. Clinical response was assessed according to Response Evaluation Criteria in Solid Tumors.13 Surgical complications were graded according to the Memorial Sloan Kettering Cancer Center grading system,14 which categorizes the complications into five grades ranked 0–1: grade 1 (absent/minor complications not requiring therapy); grade 2 (complications requiring medications only without invasive procedures); grade 3 (complications leading to lasting disability or organ resection); grade 4 (life-threatening complications requiring intensive care unit stay); and grade 5 (death due to complications). Follow-up procedures were performed in accordance with National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology.3 In the case of increased CA-125 serum levels and/or clinical suspicion of recurrence, a CT scan and/or fluorodeoxyglucose-positron emission tomography scan were requested to confirm recurrence.
Survival analysis
Progression-free survival was defined as the time elapsed from initial diagnosis to relapse or last follow-up visit. Overall survival was calculated from the time of initial diagnosis to the date of death or last follow-up visit. Median follow-up was calculated according to the inverted Kaplan–Meier technique.15 Overall survival and PFS curves were estimated by the Kaplan– Meier product limit method.16 Only women with a minimum follow-up of 6 months from interval surgery or any cancer-related event before 6 months were included in the survival analysis. All statistical calculations were performed using the Stata software version 13.0 (Stata Corp, College Station, TX).
Results
Of the 20 gynecological cancer centers asked to participate in the INTERNATIONAL MISSION study, seven centers agreed to participate and five were included, with an overall 127 consecutive patients (Figure 1). Patients were treated between July 2009 and July 2017: 94 patients (74%) were deemed to have unresectable tumor volume at primary surgery by laparoscopy, 14 patients (11%) were considered unresectable by laparotomy, and 19 patients (15%) were deemed unresectable by combined evaluation with CT scan and cytology. Patient characteristics are included in Table 1. Median age was 60 years, obesity was found in 13.8% (median body mass index 24.7 kg/m2, range 15.6–64). The histological type of ovarian cancer was high-grade serous in 90.6% of patients and most had stage IIIC disease (83.5%), with ASA score of 2 (83.5%).
Number of patients enrolled in each center.
Patient characteristics.
All patients received neoadjuvant platinum-based chemotherapy with a median number of four cycles (range 3–8). An overall response rate of 96.8% was reported (38 patients [29.9%] with a clinical complete response and 85 patients [66.9%] with a clinical partial response) according to Response Evaluation Criteria in Solid Tumors.13 At final pathological diagnosis, complete response was observed in 27 patients and partial microscopic response in 19 additional patients (Table 2). Ninety-five of 127 (72.5%) patients received adjuvant chemotherapy after interval surgery. All patients were optimally resected (residual tumor <1 cm) at the time of interval surgery:17 among them, 122 (96.1%) patients had no residual tumor. Concomitant surgical procedures performed are described in Table 3. Median operative time was 225 min (range 60–600) and median estimated blood loss was 100 mL (range 70–1320). Median time to discharge was 2 days (range 1–33) and estimated median time to start chemotherapy was 20 days (range 15–60).
Chemotherapy details
Surgical data
Among the 127 patients included, six (4.8%) had intraoperative complications, with one patient having two serious complications (bowel and bladder injury at the same time). There were five (3.9%) postoperative short-term complications, however only three had grade 3 according to the Memorial Sloan Kettering Cancer Center grading system, consisting of two bowel fistulas and one pleural effusion. The conversion rate to laparotomy was 3.9% (five of 127) and reasons for conversion were surgical adhesions in three patients, one bowel resection, and one iliac artery injury (Table 4).
Complications data
Survival analysis
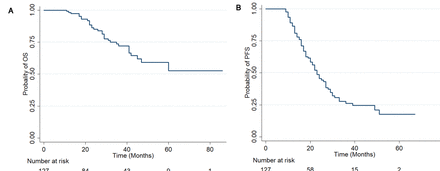
Survival analysis was performed for all patients. Median follow-up was 37 months (range 7–86): 74 patients have recurred (58.3%) and 31 (24.4%) have died from the disease. The pattern of recurrence was mainly intraperitoneal (56 of 74, 75.7%), either exclusive or mixed with other sites (parenchymal and/or lymph nodal). The median progression-free survival was 23 months and the survival at 5 years was 52.6% (95% CI: 35.2 to 67.3) (Figure 2A,B).
Progression-free (A) and overall (B) survival of patients treated with MIS after NACT, following a minimum follow-up time of 6 months after debulking or any cancer-related event before 6 months.
Discussion
Despite the increasing use of neoadjuvant chemotherapy and interval surgery in advanced epithelial ovarian cancer, recent literature contains only a few studies on the minimally invasive approaches, with limitations such as a small series of cases.11 12 18–20 An analysis of the US National Cancer Database has recently shown that patients with advanced ovarian cancer selected for laparoscopic interval surgery after neoadjuvant chemotherapy have similar perioperative outcomes and survival rates to women who undergo interval surgery by laparotomy.21 Conversely, the present study shows that minimally invasive surgery approaches are currently underused to perform interval surgery, even in some major centers with a focus on ovarian cancer treatment by laparoscopy or robotics.
Nevertheless, the current study demonstrated that minimally invasive interval surgery is a reasonable approach for patients, with advanced ovarian cancer after neoadjuvant chemotherapy who have had either complete or partial clinical response. The advantages of minimally invasive surgery compared with the traditional laparotomic technique22 are confirmed. Overall, the conversion rate of 3.9% is low, although higher than previously reported.11 This difference is probably the result of the broader inclusion criteria adopted, with no limitations in terms of body mass index, ASA score, or the number of chemotherapy cycles. The presence of extensive adhesions and gross residual disease after neoadjuvant chemotherapy, which are related to the high tumor burden at diagnosis, may also explain both conversion and complication rates in this series. Nevertheless, the safety of minimally invasive interval surgery is presented herein, with only six patients having an intraoperative complication (4.7%) and three (2.4%) patients experiencing severe early postoperative complications.
Another main goal of this study was to ensure that there was no adverse impact on patient survival. Critics may argue that a thorough peritoneal evaluation is not possible with minimally invasive surgery, resulting in undetected disease and worsened oncologic outcomes. Although eligibility to undergo interval surgery is related to response to neoadjuvant therapy, with an evident selection bias, the median progression-free and overall survival reported in this series seems reassuring regarding the oncologic safety of this approach (Figure 2).
We acknowledge that the short-term accrual and the heterogeneity of the patients, as well as their treatments in term of surgery and chemotherapy may represent potential limitations to this study. Based on the current study, we suggest starting surgery with diagnostic laparoscopy and, if feasible, safely continuing by either laparoscopy or robotics to decrease the impact of aggressive surgery on high-morbidity patients, and thus reduce time to chemotherapy.12 This approach has also been found to be cost effective and can be used as a diagnostic tool before primary cytoreductive surgery in ovarian cancer.23 With this method, patients can be identified with insufficient response to NACT despite clinical evaluation, which can therefore lead to additional cycles of chemotherapy or to second-line therapy.24
Interval debulking surgery can integrate standard treatment, assess pathological response, and provides an opportunity to remove chemotherapy-resistant clones while improving quality of life in some cases.25 Recently published data have shown an acceptable outcome without interval surgery, suggesting a marginal role in women with good response to chemotherapy.26 In this complex scenario, a minimally invasive approach may be considered in the management of patients with advanced ovarian cancer who have undergone neoadjuvant chemotherapy, when surgery is limited to low-complexity cytoreductive procedures. Minimally invasive surgery allows surgeons to reserve the use of laparotomy for higher complexity procedures that may, in turn, be associated with a higher risk of complications.
Supplemental material
Acknowledgments
The authors wish to thank the following contributors: Dr. Minig L (Department of Gynecology, Instituto Valenciano de Oncología (IVO), Valencia, Spain) and Dr. Kim TJ (Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea).
References
Footnotes
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Not commissioned; externally peer reviewed.