Abstract
Background
Sentinel lymph node (SLN) surgery is used worldwide for staging breast cancer patients and helps limit axillary lymph node dissection. [99mTc]Tilmanocept is a novel receptor-targeted radiopharmaceutical evaluated in 2 open-label, nonrandomized, within-patient, phase 3 trials designed to assess the lymphatic mapping performance.
Methods
A total of 13 centers contributed 148 patients with breast cancer. Each patient received [99mTc]tilmanocept and vital blue dye (VBD). Lymph nodes identified intraoperatively as radioactive and/or blue stained were excised and histologically examined. The primary endpoint, concordance (lower boundary set point at 90 %), was the proportion of nodes detected by VBD and [99mTc]tilmanocept.
Results
A total of 13 centers contributed 148 patients who were injected with both agents. Intraoperatively, 207 of 209 nodes detected by VBD were also detected by [99mTc]tilmanocept for a concordance rate of 99.04 % (p < 0.0001). [99mTc]tilmanocept detected a total of 320 nodes, of which 207 (64.7 %) were detected by VBD. [99mTc]Tilmanocept detected at least 1 SLN in more patients (146) than did VBD (131, p < 0.0001). In 129 of 131 patients with ≥1 blue node, all blue nodes were radioactive. Of 33 pathology-positive nodes (18.2 % patient pathology rate), [99mTc]tilmanocept detected 31 of 33, whereas VBD detected only 25 of 33 (p = 0.0312). No pathology-positive SLNs were detected exclusively by VBD. No serious adverse events were attributed to [99mTc]tilmanocept.
Conclusion
[99mTc]Tilmanocept demonstrated success in detecting a SLN while meeting the primary endpoint. Interestingly, [99mTc]tilmanocept was additionally noted to identify more SLNs in more patients. This localization represented a higher number of metastatic breast cancer lymph nodes than that of VBD.
Similar content being viewed by others
Sentinel lymph node (SLN) identification during breast surgery has been extensively validated.1 – 6 In current SLN biopsy practice, a vital blue dye (VBD) may be paired with a colloidal radiotracer with the intent to complement its performance by increasing SLN detection rate and identification accuracy.7 , 8 However, the overall efficacy of SLN identification depends heavily on the specificity of the agent(s) used in the mapping procedure in order to provide reliable localization and retention of the agent in the sentinel node(s).
[99mTc]Tilmanocept (Fig. 1) is a novel, engineered radiopharmaceutical specifically designed for lymphoscintigraphy and intraoperative SLN detection.9 [99mTc]Tilmanocept consists of multiple DTPA and mannose moieties tethered to a dextran scaffold. DTPA chelates and holds 99mTc, while its multiple mannose moieties facilitate specific multivalent binding to mannose receptors (CD206) expressed on the surfaces of reticuloendothelial cells residing within lymph nodes.10 Preclinical studies and several phase 1 and phase 2 clinical trials demonstrated that [99mTc]tilmanocept’s chemical structure and relatively small molecular size (MW = 16.7 kDa) and small molecular diameter of 7.1 nm enable [99mTc]tilmanocept to exit its injection site more rapidly than radiolabeled colloids and quickly accumulate at the SLNs, while specific multivalent interactions between its mannose moieties and CD206 enable avid binding to target receptors and retention in SLNs for up to 30 h, without observed transit to second-echelon lymph nodes.9 – 15
[99mTc]Tilmanocept is composed of a dextran backbone (black) to which are attached multiple units of mannose (green) and DTPA (blue). The mannose units provide a molecular mechanism by which [99mTc]tilmanocept avidly binds to a receptor specific to recticuloendothelial cells (CD206), and the DTPA units provide a highly stable means to radiolabel tilmanocept with technetium-99m (red). The molecular weight of [99mTc]tilmanocept is approximately 17,000 grams per mole; the molecular diameter is 7.1 nm
The clinical trials reported here were designed in consultation with the US Food and Drug Administration and the European Medicine Agency for the application of [99mTc]tilmanocept for approval as a new drug. Here, we report the results from 2 highly similar prospective, phase 3 clinical trials of [99mTc]tilmanocept in patients undergoing SLN mapping for breast cancer. The results from the melanoma patients are reported separately in a companion paper.16 On March 13, 2013 the US Food and Drug Administration approved [99mTc]tilmanocept for intraoperative lymph node mapping.
Materials and Methods
Study Design
From June 2008 to April 2011, 2 highly similar phase 3 trials were performed on breast cancer patients without presurgical evidence of lymph node metastases who were scheduled for surgeries that included clinically indicated SLN mapping. The primary aim of both studies was to evaluate the concordance of [99mTc]tilmanocept with VBD, a mapping agent that is routinely used to identify SLNs. The studies were designed as prospective, phase 3, open-label, nonrandomized, within-patient trials in which all patients received both [99mTc]tilmanocept and a VBD (Lymphazurin). The first trial involved 8 centers enrolling at least 1 breast cancer patient between June 2008 and June 2009. A second trial was conducted to extend the safety database to 500 subjects (including phase 1 and 2 trials). The second trial involved 5 sites enrolling breast cancer patients from July 2010 to April 2011 and used the same study design and procedures. These studies were powered at 0.8 or greater for the primary endpoint. All patients gave written informed consent. The Western Institutional Review Board and institutional review boards at each enrolling institution approved the protocol, patient instructions, and informed consent documentation. Both studies complied with all provisions of the Declaration of Helsinki and US laws requiring registration and updates via ClinicalTrials.gov (trial No. NCT00671918 and NCT01106040). This report describes results obtained from the breast cancer patients who participated in these studies.
Enrollment criteria included the histologically confirmed presence of unilateral breast cancer with a surgical treatment plan that included SLN mapping. The complete list of criteria is presented in Table 1. The studies defined 3 populations of patients: a population of all enrolled patients, an intent-to-treat (ITT) population, and the safety population. The ITT population consisted of all enrolled patients receiving both [99mTc]tilmanocept and VBD and who had at least one histologically confirmed lymph node stained by VBD. This was the population in which the concordance of [99mTc]tilmanocept to VBD could be assessed, hence the ITT designation. Analyses of pathology rates were based on findings from any patient injected with [99mTc]tilmanocept and from whom lymph nodes were removed. Drug safety was based on the safety population, consisting of all patients who received [99mTc]tilmanocept whether or not they received VBD.
Site qualification required that all participating surgeons and investigators had performed at least 30 SLN procedures with a radiopharmaceutical within the past 90 days prior to initiation of the trial.
Procedures
Patients received 3.0 nmol of [99mTc]tilmanocept (50 μg). Patients scheduled for surgery on the same day as the injection received ~0.5 mCi of [99mTc]tilmanocept. Patients scheduled for “next-day” surgery received ~1.0–2.0 mCi of [99mTc]tilmanocept. The radiopharmaceutical was administered using one of the following routes of administration: intradermal, subareolar, or peritumoral. For a given patient, isosulfan blue was injected after [99mTc]tilmanocept at the time of surgery using the same injection route as the radiopharmaceutical. Injections of [99mTc]tilmanocept were not accompanied by either topical application of or coinjection with local anesthetics.
Intraoperative identification of SLNs was based on three criteria. The first criterion was visual identification of VBD in a node and/or the afferent lymphatic channel; all blue-stained nodes were removed and designated as “blue.” Observation of radioactivity in a lymph node, indicating the localization of [99mTc]tilmanocept, formed the basis of the second criterion. Background radioactivity levels were measured using a handheld intraoperative gamma detector/probe (programmed to read out in counts per second) at the skin surface well away (not less than 20 cm) from the injection site. The background count rate was recorded directly from the display of the gamma detection system. The standard deviation of the background was calculated as the square root of the total number of counts acquired during the measurement, which equaled the background count rate, in counts per second, times the duration of the counting time, in seconds. To qualify as a hot node, the intraoperative counts in the node had to exceed the background count (using either one 10-second count or the average of three 2-second counts, with background measured directly on the patient ≥20 cm from the primary site) plus 3 times the standard deviation of the background and exceed 25 counts per second. The “3-sigma rule,” was selected for this study based on statistical rigor; using this rule provides 99.7 % certainty that detection of the “hot” node did not occur due to chance. Blue nodes were evaluated for radioactivity prior to and after excision. The third SLN identification criterion was based on appearance and feel; visibly or palpably abnormal lymph nodes were designated as palpable masses and were excised regardless of agent localized. Mapping was considered complete when all nodes meeting any of the 3 criteria had been removed and all remaining nodes in the basin were designated as negative based on these criteria. All excised nodes and tissues underwent histopathological evaluation that included hematoxylin and eosin staining and immunohistochemical analysis. Based on tissue analysis, tissue organization was confirmed (lymph node) and pathology status was determined.
Safety Data
The safety endpoint was monitored by performing follow-up safety labs, EKGs, and physical examinations 6–30 h postinjection and comparing results with baseline values. All patients were monitored for adverse events. Adverse events were considered severe if they resulted in death, represented a life-threatening reaction, or required prolonged or readmission to the hospital. The principal investigator for each site was responsible for determining if adverse events were definitely not, probably not, possibly, probably, or definitely related to [99mTc]tilmanocept administration. The safety data was analyzed with parametric statistics if the test results were reported as continuous variables.
Statistical Plan
The primary efficacy endpoint in both studies was the concordance of radioactive lymph nodes identified with [99mTc]tilmanocept and those identified with VBD. Concordance of [99mTc]tilmanocept with VBD was defined as the number of blue-stained nodes that were detected by [99mTc]tilmanocept, divided by the number of blue-stained lymph nodes. A supportive secondary endpoint was the patient concordance rate defined as the number of patients for whom all nodes detected by VBD were also detected by [99mTc]tilmanocept, divided by the number of patients with at least one blue-stained lymph node (ITT population). Other secondary efficacy endpoints included the proportions of all removed lymph nodes that were detected by [99mTc]tilmanocept and/or VBD. The proportions of pathology-positive lymph nodes that were blue and/or radioactive were evaluated, including calculation of failed detection rates for the imaging agent. Concordance (R) was tested in a pooled analysis with the hypothesis H0: R ≤ 0.90 versus Ha: R > 0.90. A 95 % confidence interval for the concordance rate was calculated using a large sample normal approximation.
Role of the Funding Source
Navidea Biopharmaceuticals (Dublin, OH) sponsored these trials and supplied tilmanocept kits for radiolabeling to each study site. STATKING Clinical Services (Fairfield, OH), an independent data analysis group, facilitated independent data auditing and analyses.
RESULTS
Patient Population
A total of 152 women with breast cancer were enrolled in the 2 trials, and 149 were injected with [99mTc]tilmanocept (3 patients withdrew prior to injection). Therefore, 149 made up the safety population (all patients receiving [99mTc]tilmanocept). All breast cancer patients were female. The safety population’s average age was 58.0 years (range, 31–84 years). Specific demographics of the various subpopulations of the safety population are listed in Table 2. One patient injected with [99mTc]tilmanocept was not injected with VBD. All surgeons involved in both trials had at least 10 years of experience performing sentinel lymph node mapping of breast cancer.
Intraoperative Node Identification
There were 148 patients injected with both [99mTc]tilmanocept and VBD and who underwent surgery, of which 131 (88.5 %) had at least one blue node (ITT population) and 146 (98.6 %) had at least one radioactive node (Table 3). The observation that [99mTc]tilmanocept detected SLNs in more patients than VBD (98.6 vs 88.5 %) was statistically significant (p < 0.0001). A single breast cancer patient had two palpable lymph nodes missed upon pre-enrollment review (later found to contain cancer) that were neither blue-stained nor radioactive. Prospectively, per protocol, these nodes were defined as SLNs.
There were 326 lymph nodes examined in these studies. Of 131 patients with at least 1 blue node (ITT population), an average of 1.60 blue nodes were detected per ITT patient for a total of 209 blue nodes. Of 146 patients with at least one radioactive node, an average of 2.16 radioactive nodes were detected per patient for a total of 320 radioactive nodes. Also, 15 patients with at least one radioactive node had no blue nodes.
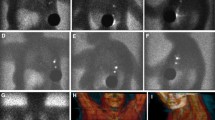
Figure 2 is a fused sagittal cross section acquired by SPECT/CT imaging at 1 h postinjection. The cross section visualizes a sentinel lymph node (arrow) and the injection site. At 5 h after injection, 3 blue and hot lymph nodes (6,724 cps at arrow, 1,477 cps, 167 cps) were detected at surgery and excised. Pathologic examination revealed 1 pathology positive lymph node (blue with 6,724 cps, 1.7 × 1.3 × 0.7 cm) and 2 pathology negative lymph nodes.
Lymphoscintigraphy of a 35-year-old woman with carcinoma in situ of the left breast showing 2 intense foci of noted [99mTc]tilmanocept localization within the left axilla. An intradermal injection (0.4 mL, 0.5 mCi, 3.0 nmol) of [99mTc]tilmanocept was administered to the upper left quadrant of the left breast. The SPECT/CT image is a fused sagittal cross section acquired 1 h postinjection, which visualizes a sentinel lymph node (arrow) and the injection site. At 5 h after injection, 3 blue and hot lymph nodes (6,724 cps, 1,477 cps, 167 cps) were detected at surgery and excised. Pathologic examination revealed 1 histologically positive lymph node (blue with 6,700 cps, 1.7 × 1.3 × 0.7 cm) and 2 negative lymph nodes
Concordance of [99mTc]Tilmanocept and Vital Blue Dye
The primary efficacy endpoint in both studies was the concordance of radioactive lymph nodes identified with [99mTc]tilmanocept and those identified with VBD. Of 209 blue lymph nodes identified in the ITT population, 207 (99.04 %, 95 % CI = 96.59–99.88 %) were also radioactive (Table 4). This concordance rate was statistically significant (p < 0.0001), convincingly rejecting the null hypothesis of ≤90 %. For 129 of 131 patients in the ITT population, all blue nodes were also radioactive for a pooled patient concordance rate of 98.47 %.
Pathology Findings
Of the 148 injected with both [99mTc]tilmanocept and VBD and who underwent surgery, a subset analysis was performed on the 27 patients in which 1 or more pathology-positive lymph nodes were detected (Table 3). A total of 33 pathology-positive nodes were detected in these 27 patients. All metastases were identified by hematoxylin and eosin staining and were greater than 2 mm. Except for 2 cancer-containing palpable lymph nodes found in 1 patient in the first study (mentioned previously), all pathology-positive lymph nodes were radioactive. Conversely, while [99mTc]tilmanocept detected 31 of 33 cancer-containing lymph nodes (93.9 %), VBD identified only 25 of 33 such nodes (75.8 %), missing the involved pathology-positive lymph node(s) in 6 patients (22.2 %). In fact, 2 patients (2 of 148; 1.35 %) had their nodal disease status upstaged by [99mTc]tilmanocept findings alone; no patients were upstaged by VBD alone. Thus, [99mTc]tilmanocept exhibited a higher detection rate for disease-positive lymph nodes than VBD on a per node and per patient basis. The nodal failed detection rate over both studies for [99mTc]tilmanocept was 6.06 %, whereas VBD’s nodal failed detection rate was a significantly greater at 24.24 % (p = 0.0312).
Adverse Events
No patient deaths were reported on study. A total of 74 adverse events occurred in 49 patients in the safety population of breast cancer patients receiving [99mTc]tilmanocept (N = 149). The most common events included nausea, seroma, and urinary tract infection. There were 3 serious adverse events reported from 3 patients (modified radical mastectomy, herpes zoster ophthalimic, and cellulitis), none of which was deemed “probably” or “definitely” related to [99mTc]tilmanocept. The remaining adverse events were generally considered mild. There were no immediate or delayed hypersensitivity reactions to [99mTc]tilmanocept. None of the events that were considered clinically significant was related to the administration of [99mTc]tilmanocept. Even though local analgesics were not used in conjunction with administration of the [99mTc]tilmanocept, only 2 patients reported injection site pain or slight breast pain during injection related to study drug. Both of these adverse events were considered mild and resolved the same day as onset.
DISCUSSION
In this pooled analysis of breast cancer patients participating in 2 highly similar phase 3 trials, [99mTc]tilmanocept’s identification of lymph nodes was highly concordant with identification by VBD. Of the 209 lymph nodes identified by VBD, 207 were also identified by [99mTc]tilmanocept (p < 0.0001). Interestingly, VBD failed to identify a SLN in 17 of 148 breast cancer patients (11.5 %). This was less than the reported failure rate for VBD.17 Within these 17 patients, [99mTc]tilmanocept identified at least 1 node in all but 2; notably, these patients also had no blue nodes. There were significantly (p < 0.0001) more radioactive nodes (SLN = 320) than blue nodes (SLN = 209). Of 320 radioactive nodes, only 207 (64.69 %) were also blue. Similarly, in only 78 of 146 patients (53.42 %) with at least 1 radioactive node were all the radioactive nodes also blue. Our findings are consistent with those of Krag and coworkers, where the isotope was better than VBD in all aspects.18
[99mTc]Tilmanocept’s ability to identify more cancer-containing nodes than VBD (31 vs. 25, p < 0.0312) demonstrates that [99mTc]tilmanocept provides a benefit in intraoperative lymphatic mapping beyond that provided by VBD alone. Recent reports that local recurrence and survival are not affected by full node dissection after positive sentinel node biopsy in breast conservation patients accentuates the importance of the performance of the [99mTc]tilmanocept SLN mapping agent in these studies and points to the need to accurately assess lymph nodes in order to optimize postsurgery management of the patients.1 , 19 , 20 Our findings suggest, then, there is significant clinical utility in knowing which patients have multiple positive sentinel nodes.
Entrance of a patient into the clinical trial was at the discretion of the surgeon. As long as the patient did not have metastatic disease, clinically positive lymph nodes, or any of the exclusion criteria listed in Table 1, the patient was eligible for the study. If the 2 T4 patients were excluded the concordance would be slightly higher and the performance of VBD would appear to be better. This is because in one of the patients VBD failed to stain a disease-positive lymph node. Two patients were stage T4. Both patients had 2 “hot” lymph nodes. Both nodes of one patient were disease-positive with 1 of the lymph nodes not stained blue. The other patient had a “hot” and “blue-stained” lymph node that was disease-positive; the second “hot” lymph node was disease-negative and not stained blue. Based on criteria other than T staging, for example, N0, M0, there is no reason to exclude such patients.
There is a growing discussion as to whether or not a complete node dissection is necessary.3 , 19 , 21 [99mTc]Tilmanocept identified numbers of patients who had additional pathology-positive sentinel nodes, while VBD did not. A study site, University of California, San Diego, had 20 breast cancer patients with pathology-positive nodes identified with at least 1 radioactive and/or blue sentinel node; 16 had only a single radioactive and blue positive sentinel node. Also, four patients had additional radioactive pathology-positive nodes that were not blue, deeming them “multinode positive patients.” The indication that [99mTc]tilmanocept may be able to better identify multinode pathology-positive patients may be important to the future of breast cancer treatment. Specifically, extended field radiation therapy will be offered to these patients if not full node dissection. To the extent that [99mTc]tilmanocept accumulates at that the target tissue based on a specific biochemical interaction (CD206 receptor-targeted), this molecule represents the current generation of imaging technology and is specifically designed to match the biochemical requirements of the clinical problem. Thus, the increase in found disease represents a notable finding related to the SLN procedure, especially where this is translated to a large patient population. If blue dye alone were used, the addition of tilmanocept could potentially change the diagnosed number of positive nodes in 1.35 % of our nation’s 190,000 or so breast cancer patients, which is roughly 2,000 patients per year. Since adjuvant decisions are now often based on sentinel node biopsy results alone, accurately knowing those patients who have multiple positive nodes may change radiation and systemic therapy choices.
Vital blue dye was chosen as the comparator in these studies because it is routinely used in current practice to identify SLNs during breast cancer surgery and has a history as a validation agent for SLN biopsy.6 , 22 Radioactive colloids have been used off-label with the intent to augment VBD.7 , 8 , 23 However, [99mTc] sulfur colloid injection (SCI) has recently been granted FDA approval in the United States for lymphatic mapping during breast cancer operations only. Nevertheless, radiocolloids remain an unstandardized SLN mapping agent, mitigating their utility as a valid comparator agent. VBD usage permitted a rigorous, within-patient experimental design, significantly increasing the statistical power. There is a possibility despite finding one blue node and finding several hot nodes, that a rare additional aberrant blue node was missed somewhere. It is also possible when no blue node was identified at all and several hot nodes were found, that again a distant blue node may have been overlooked. However, most of the positive axilla did go on to full node dissection, and no further blue nodes were identified.
These phase 3 studies provided a basis with which to compare the performance of the radiolabeled colloids via meta-analysis. A recent review of SCI was based on a meta-analysis of 15 studies involving 9,213 breast cancer patients receiving both SCI and VBD.24 In the meta-analysis, SCI and VBD detected at least 1 lymph node in 94.1 and 85.1 % of patients, respectively. In the current studies, [99mTc]tilmanocept and VBD detected at least 1 lymph node in 98.6 % (146 of 148) and 88.5 % (131 of 148) of patients, respectively. Lastly, we constructed a meta-analysis of these phase 3 trials and contrasted the results against the recently published selected performance data of Nanocoll.25 Tilmanocept had superior SLN localization rate (99.9 vs. 95.1 %) and a higher degree of localization (2.16 vs. 1.66 SLN per study) (p < 0.0001). Such comparisons will be duplicated with larger sample sizes if [99mTc]tilmanocept becomes commercially available.
These phase 3 trial and the previous phase 1 and 3 studies provided preliminary data regarding the performance of [99mTc]tilmanocept compared with the current SLN agents. First, [99mTc]tilmanocept is a wholly standardized synthetic molecule with a defined molecular structure that permits rapid uptake into lymph nodes. When imaged within 10 min postinjection, at least 1 “hot” lymph node was detected in 98 % of the early images. Second, based on physician use reports, the administration of [99mTc]tilmanocept is associated with significantly less pain and discomfort than for radiolabeled colloids. Third, based on the contrasts of dosimetry and preoperative imaging, [99mTc]tilmanocept exits its injection site significantly quicker than radiolabeled colloids, creating less shine-through, thus facilitating better step-down for observation of SLNs that reside near injection sites.15 Finally, both in vivo imaging and in vitro analysis of [99mTc]tilmanocept’s receptor binding properties indicate it binds specifically to SLNs and does not move downstream to distal lymph nodes, permitting high confidence that a “hot” lymph node found during a “next-day” surgery is a sentinel lymph node.9 , 15 , 26 This factor also allows the patient to be imaged as early as 10 min and as late as 24 h or more after injection. These attributes will translate into greater scheduling flexibility in nuclear medicine and operating room without sacrificing either short-term or long-term SLN detection success.
[99mTc]Tilmanocept is a modern radiopharmaceutical designed on a chemical platform that permits future development.26 Technetium-99m-labeled antimony sulfide colloid and sulfur colloid, both of which were developed more than 35 years ago, cannot be chemically modified.27 , 28 The chemical design of tilmanocept permits the facile attachment of additional imaging reporters, such as fluorescent dyes.29 , 30 Routine optical imaging via robotic-assistance or intraoperative hand-held imaging will require the covalent attachment of the fluorescent tag to the molecular imaging agent.31 , 32 Optical imaging would facilitate intraoperative administration, which may be more compatible with SLN mapping of lung, GI, and GU cancers. The DTPA chelators of tilmanocept (Fig. 1) permit radiolabeling with different radioisotopes, such as gallium-68 or indium-111 or paramagnetic atoms.33 Gallium-68, which is a generator-produced positron-emitter, enables PET/CT hybrid imaging.34 PET imaging provides higher sensitivity and spatial resolution, as well as greater scatter and attenuation correction than SPECT. Hybrid imaging with PET/CT may prove to be more compatible for preoperative pelvic imaging of prostate cancer. Lastly, Nanocoll is a microcolloid of human serum albumin and must be tested for antibodies to HIV and hepatitis C, as well as hepatitis B surface antigen.35 Rigorous testing and increased regulatory compliance add expense to the manufacturing of Nanocoll and substantially decreases the likelihood that modifications for performance improvements will be commercialized. Tilmanocept is not derived from blood and therefore can be synthesized at very high scales without continual monitoring for new biological “threats” from the human blood supply.
There were two reasons for using the “3-sigma” rule. First, the “3-sigma” rule is based on a statistical rationale and is used extensively in the radiation sciences to define a criteria known as the “Minimum Detectable Activity.”36 When a measurement is greater than background by 3 times the standard deviation of the background, the probability that the measurement is truly different than background is greater than 99.7 %. The measurement of radioactivity is a counting process that follows a Poisson distribution, which occurs when the process is the result of many attempts and very few successes. The standard deviation of a Poisson distribution is the square root of the measurement. Consequently, the Minimum Detectable Activity for a sentinel lymph node is 3 times the square root of the background count rate, which can be obtained by a single measurement. Second, the “10 %” was empirically established for radiocolloids and may not be applicable to a nonparticulate receptor-targeted radiopharmaceutical.18 Due to its small size (7 nm) and high receptor avidity, tilmanocept delivers significantly more radioactivity to the sentinel lymph node and clears from the injection site 5-fold faster than sulfur colloid. For example, the UCSD breast cancer patients were studied with a protocol that used a single intradermal injection of 0.1 ml. A total of 95 patients had at least 1 “hot” lymph node. The count rate of the “hottest” lymph node from each patient ranged from 375 cps to an excess of 999,999 cps, which is the readout maximum of the Neo200 counting system. Seven lymph nodes exceeded this count rate. The median count rate of the “hottest” lymph node was 15,738 cps. Appling the “10 % rule” when the “hottest” node has 15,000 cps would permit the surgeon to stop if the surgical bed had a count rate of 1,500 cps or less. This would risk leaving behind a lymph node that could be 1,500 cps and by radiocolloid standards would be considered very “hot.” Such a lymph node would be almost 3 orders-of-magnitude higher than background and would more than satisfy the “3-sigma rule,” which was our statistical basis for defining a radioactive signal and, hence, a sentinel lymph node.
A sentinel lymph node definition based on the technical criteria of “Minimal Detectable Activity” may not provide the most efficient “stopping rule” for the mapping procedure. Although a “stopping rule” for a receptor-targeted agent such as [99mTc]tilmanocept will require a separate study and may not be at all similar to the rule used for particulate radiotracers, a comparison of our results to the “10 % rule,” which is used for unfiltered and filtered [99mTc]labeled sulfur colloid may be instructive.18 , 37 – 40 The “10 % rule” and the “3-sigma rule” identified the same number of patients (n = 146) with at least 1 “hot” lymph node. Consequently, the per-patient concordance rate was the same using both rules. Additionally, defining the sentinel node by the “10 % rule” did not alter the pathology status on a per-patient basis. Also, 17 fewer sentinel lymph nodes were defined as “hot” based on the “10 % rule.” Of these, 11 nodes were blue, and 2 of the 11 were pathology-positive.
In summary, these studies demonstrated that SLN detection by [99mTc]tilmanocept is highly concordant to SLN detection by VBD. [99mTc]Tilmanocept detected more SLNs than VBD, and these additional SLNs enhanced the detection of clinically important nodal metastases. Given these results, this agent is safe and provides a modern radiopharmaceutical for future advances in sentinel lymph node mapping.
References
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75.
Gipponi M, Bassetti C, Canavese G, Catturich A, Di Somma C, Vecchio C, et al. Sentinel lymph node as a new marker for therapeutic planning in breast cancer patients. J Surg Oncol. 2004;85:102–11.
Krag DN. Current status of axillary lymph node dissection and sentinel node biopsy in breast cancer. Clin Adv Hematol Oncol. 2010;8:471–3.
Rodier J, Janser J. Surgical technical details improving sentinel node identification in breast cancer. Oncol Rep. 1997;4:281–3.
Newman LA. Lymphatic mapping and sentinel lymph node biopsy in breast cancer patients: a comprehensive review of variations in performance and technique. J Am Coll Surg. 2004;199:804–16.
Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg. 1997;226:271–8.
Martin RC, 2nd, Edwards MJ, Wong SL, Tuttle TM, Carlson DJ, Brown CM, et al. Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. For the University of Louisville Breast Cancer Study Group. Surgery. 2000;128:139–44.
Cody HS, Fey J, Akhurst T, Fazzari M, Mazumdar M, Yeung H, et al. Complementarity of blue dye and isotope in sentinel node localization for breast cancer: univariate and multivariate analysis of 966 procedures. Ann Surg Oncol. 2001;8:13–9.
Vera DR, Wallace AM, Hoh CK, Mattrey RF. A synthetic macromolecule for sentinel node detection: [99mTc]DTPA-mannosyl-dextran. J Nucl Med. 2001;42:951–9.
Ezekowitz RA, Sastry K, Bailly P, Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990;172:1785–94.
Wallace AM, Hoh CK, Vera DR, Darrah D, Schulteis G. Lymphoseek: a molecular radiopharmaceutical for sentinel node detection. Ann Surg Oncol. 2003;10:531–8.
Leong SPL, Kim J, Ross MI, Faries M, Scoggins CR, Metz WL, et al. A phase 2 study of [99mTc]tilmanocept in the detection of sentinel lymph nodes in melanoma and breast cancer. Ann Surg Oncol. 2011;18:961–9.
Wallace AM, Hoh CK, Ellner SJ, Darrah DD, Schulteis G, Vera DR. Lymphoseek: a molecular imaging agent for melanoma sentinel lymph node mapping. Ann Surg Oncol. 2007;14:913–21.
Wallace AM, Hoh CK, Darrah DD, Schulteis G, Vera DR. Sentinel lymph node mapping of breast cancer via intradermal administration of Lymphoseek. Nucl Med Biol. 2007;34:849–53.
Wallace AM, Hoh CK, Limmer KK, Darrah DD, Schulteis G, Vera DR. Sentinel lymph node accumulation of Lymphoseek and Tc-99m-sulfur colloid using a “2-day” protocol. Nucl Med Biol. 2009;36:687–92.
Sondak VK, King DW, Zager JS, Schneebaum S, Kim J, Leong SP, et al. Combined analysis of phase III trials evaluating [99mTc]tilmanocept and vital blue dye for identification of sentinel lymph nodes in clinically node-negative cutaneous melanoma. Ann Surg Oncol. 2013;20:680–8.
Faries MB, Morton DL. Surgery and sentinel lymph node biopsy. Semin Oncol. 2007;34:498–508.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–8.
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–32; discussion 432–3.
Rayhanabad J, Yegiyants S, Putchakayala K, Haig P, Romero L, Difronzo LA. Axillary recurrence is low in patients with breast cancer who do not undergo completion axillary lymph node dissection for micrometastases in sentinel lymph nodes. Am Surg. 2010;76:1088–91.
Weaver DL, Ashikaga T, Krag DN, Skelly JM, Anderson SJ, Harlow SP, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364:412–21.
Morton DL, Bostick PJ. Will the true sentinel node please stand? Ann Surg Oncol. 1999;6:12–4.
Meyer-Rochow GY, Martin RC, Harman CR. Sentinel node biopsy in breast cancer: validation study and comparison of blue dye alone with triple modality localization. ANZ J Surg. 2003;73:815–8.
Cope FO, Metz WL, Potter B, Abbruzzese B, Blue M, Shuping J, et al. The novel receptor targeted (CD206) 99mTc-labeled tilmanocept versus the currently employed 99mTc-sulfur colloid in intraoperative lymphatic mapping (ILM) on key performance metrics in breast cancer. J Clin Oncol. 2012;30(15 suppl):e21066.
Tokin CA, Cope FO, Metz WL, Blue MS, Potter BM, Abbruzzese BC, et al. The efficacy of tilmanocept in sentinel lymph node mapping and identification in breast cancer patients: A comparative review and meta-analysis of the 99mTc-labeled nanocolloid human serum albumin standard of care. Clin Exp Metastasis. 2012;29:681–6.
Vera DR, Hoh CK, Hall DJ, Tokin CA, Wallace AM. [99mTc]Tilmanocept: A synthetic receptor-targeted molecule for sentinel lymph node mapping. Radiopharmaceuticals for Sentinel Lymph Node Detection. Vienna: International Atomic Energy Agency (in press).
Ege GN. Internal mammary lymphoscintigraphy—the rationale, technique, interpretation and clinical application: a review based on 848 cases. Radiology. 1976;118:101–7.
Larson SM, Nelp WB. Radiopharmacology of a simplified technetium-99m-colloid preparation for photoscanning. J Nucl Med. 1966;7:817–26.
Ting R, Aguilera TA, Crisp JL, Hall DJ, Eckelman WC, Vera DR, et al. Fast 18F labeling of a near-infrared fluorophore enables positron emission tomography and optical imaging of sentinel lymph nodes. Bioconjug Chem. 2010;21:1811–9.
Emerson DK, Limmer KK, Hall DJ, Han SH, Eckelman WC, Kane CJ, et al. A receptor-targeted fluorescent radiopharmaceutical for multi-reporter sentinel lymph node imaging. Radiology. 2012;265:186–93.
van der Poel HG, Buckle T, Brouwer OR, Olmos RAV, van Leeuwen FWB. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodel tracer. Eur Urol. 2011;60:826–33.
Kawaguchi Y, Ishizawa T, Masuda K, Sato S, Kaneko J, Aoki T, et al. Hepatobiliary surgery guided by a novel fluorescent imaging technique for visualizing hepatic arteries, bile ducts, and liver cancers on color images. J Am Coll Surg. 2011;212:e33–9.
Sirlin CB, Vera DR, Corbeil JA, Caballero MB, Buxton RB, Mattrey RF. Gadolinium-DTPA-dextran: a macromolecular MR blood pool contrast agent. Acad Radiol. 2004;11:1361–9.
Stroup SP, Kane CJ, Farchshchi-Heydari S, James CM, Davis CH, Wallace AM, et al. Preoperative sentinel lymph node mapping of the prostate using PET/CT fusion imaging and Ga-68-labeled tilmanocept in a dog model. Clin Exp Metastasis. 2012;29:673–80.
Summary of product characteristics for nanocoll, kit for radiopharmaceutical preparation. Milan: GE HEALTHCARE S.R.L.; 2009.
Cherry SR, Sorenson JA, Phelps ME. Physics in Nuclear Medicine. 3rd ed. Philadelphia: Saunders; 2003.
Krag DN, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer—a multicenter validation study. N Engl J Med. 1998;339:941–6.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33.
McMasters KM, Tuttle T, Carlson D, Brown CM, Noyes RD, Glaser RL, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560–6.
Liu LC, Lang JE, Jenkins T, Lu Y, Ewing CA, Hwang SE, et al. Is it necessary to harvest additional lymph nodes after resection of the most radioactive sentinel lymph node in breast cancer? J Am Coll Surg. 2008;207:853–8.
ACKNOWLEDGMENT
The clinical trials described herein were supported by Navidea Biopharmaceuticals (Dublin, OH). Other contributors of study patients include Barbara A. Michna, MD, of the Alabama Center for Medical Research, Alabama City, AL, and Alice P. Chung, MD, of the John Wayne Cancer Institute, Santa Monica, CA.
Disclosures
David R. Vera is the inventor of tilmanocept; the remaining authors report no commercial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Wallace, A.M., Han, L.K., Povoski, S.P. et al. Comparative Evaluation of [99mTc]Tilmanocept for Sentinel Lymph Node Mapping in Breast Cancer Patients: Results of Two Phase 3 Trials. Ann Surg Oncol 20, 2590–2599 (2013). https://doi.org/10.1245/s10434-013-2887-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-013-2887-8