Abstract
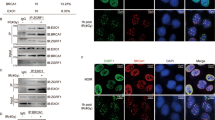
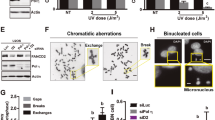
In response to DNA breaks, the ‘DNA damage response’ provokes a cell cycle arrest to facilitate DNA repair. Recent findings have indicated that cells can respond to DNA damage throughout the cell cycle, except during mitosis. Specifically, various mitotic kinases, including Cdk1, Aurora A and Plk1, were shown to inactivate key DNA damage checkpoint proteins when cells enter mitosis. Aberrant activation of mitotic kinases during interphase could therefore modulate cellular responses to DNA damage. In this study, our aim was to determine how aberrant activation of Cdk1 affects the cellular responses to DNA damage. We used Wee1 inhibition, using MK-1775, to force Cdk1 activation, which did not cause cytotoxicity in non-transformed cells. Instead, it accelerated mitotic entry and caused radio sensitization in p53-defective cancer cells, but not in p53-proficient cancer cells. Interestingly, we showed that Wee1 inhibition leads to elevation of Cdk1 activity in interphase cells. When we subsequently analyzed DNA damage responses in cells with forced Cdk1 activation, we observed a marked reduction of 53BP1 at sites of DNA damage along with an increase in γ-H2AX staining after irradiation, indicative of defective DNA repair. Indeed, when DNA repair was analyzed using in vivo endonuclease-induced homologous recombination (HR) assays, compromised DNA repair after Wee1 inhibition was confirmed. This defect in HR was accompanied by increased phosphorylation of BRCA2 at the Cdk1 phosphorylation site S3291. Taken together, our results indicate that Wee1 inhibition leads to forced Cdk1 activation in interphase cells, which interferes with normal DNA damage responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Morgan DO . Principles of CDK regulation. Nature 1995; 374: 131–134.
Kastan MB, Bartek J . Cell-cycle checkpoints and cancer. Nature 2004; 432: 316–323.
Coleman TR, Dunphy WG . Cdc2 regulatory factors. Curr Opin Cell Biol 1994; 6: 877–882.
Matsuoka S, Huang M, Elledge SJ . Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 1998; 282: 1893–1897.
Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H . Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 1997; 277: 1501–1505.
Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T . Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature 1998; 395: 507–510.
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993; 75: 817–825.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ . The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993; 75: 805–816.
Vousden KH, Lane DP . p53 in health and disease. Nat Rev Mol Cell Biol 2007; 8: 275–283.
Johnson RD, Liu N, Jasin M . Mammalian XRCC2 promotes the repair of DNA double-strand breaks by homologous recombination. Nature 1999; 401: 397–399.
Huertas P, Cortes-Ledesma F, Sartori AA, Aguilera A, Jackson SP . CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature 2008; 455: 689–692.
Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 2004; 431: 1011–1017.
Johnson N, Li YC, Walton ZE, Cheng KA, Li D, Rodig SJ et al. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med 2011; 17: 875–882.
Mailand N, Bekker-Jensen S, Bartek J, Lukas J . Destruction of Claspin by SCFbetaTrCP restrains Chk1 activation and facilitates recovery from genotoxic stress. Mol Cell 2006; 23: 307–318.
Mamely I, van Vugt MA, Smits VA, Semple JI, Lemmens B, Perrakis A et al. Polo-like kinase-1 controls proteasome-dependent degradation of Claspin during checkpoint recovery. Curr Biol 2006; 16: 1950–1955.
van Vugt MA, Gardino AK, Linding R, Ostheimer GJ, Reinhardt HC, Ong SE et al. A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1, and Chk2 to inactivate the G(2)/M DNA damage checkpoint. PLoS Biol 2010; 8: e1000287.
Dominguez-Kelly R, Martin Y, Koundrioukoff S, Tanenbaum ME, Smits VA, Medema RH et al. Wee1 controls genomic stability during replication by regulating the Mus81-Eme1 endonuclease. J Cell Biol 2011; 194: 567–579.
Hirai H, Arai T, Okada M, Nishibata T, Kobayashi M, Sakai N et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol Ther 2010; 9: 514–522.
Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, Kobayashi M et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther 2009; 8: 2992–3000.
Rajeshkumar NV, De Oliveira E, Ottenhof N, Watters J, Brooks D, Demuth T et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res 2011; 17: 2799–2806.
Nurse P . Universal control mechanism regulating onset of M-phase. Nature 1990; 344: 503–508.
Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 1997; 278: 1957–1960.
Cerqueira A, Santamaria D, Martinez-Pastor B, Cuadrado M, Fernandez-Capetillo O, Barbacid M . Overall Cdk activity modulates the DNA damage response in mammalian cells. J Cell Biol 2009; 187: 773–780.
Scully R, Xie A . In my end is my beginning: control of end resection and DSBR pathway 'choice' by cyclin-dependent kinases. Oncogene 2005; 24: 2871–2876.
van Vugt MA, Yaffe MB . Cell cycle re-entry mechanisms after DNA damage checkpoints: giving it some gas to shut off the breaks!. Cell Cycle 2010; 9: 2097–2101.
Seluanov A, Mittelman D, Pereira-Smith OM, Wilson JH, Gorbunova V . DNA end joining becomes less efficient and more error-prone during cellular senescence. Proc Natl Acad Sci USA 2004; 101: 7624–7629.
Adams BR, Hawkins AJ, Povirk LF, Valerie K . ATM- independent, high-fidelity nonhomologous end joining predominates in human embryonic stem cells. Aging 2010; 2: 582–596.
Pierce AJ, Johnson RD, Thompson LH, Jasin M . XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev 1999; 13: 2633–2638.
Crescenzi E, Palumbo G, Brady HJ . Roscovitine modulates DNA repair and senescence: implications for combination chemotherapy. Clin Cancer Res 2005; 11: 8158–8171.
Parvin J, Chiba N, Ransburgh D . Identifying the effects of BRCA1 mutations on homologous recombination using cells that express endogenous wild-type BRCA1. J Vis Exp 2011; 48: e2468.
Leijen S, Beijnen JH, Schellens JH . Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase results in sensitization of p53-deficient tumor cells to DNA-damaging agents. Curr Clin Pharmacol 2010; 5: 186–191.
van Vugt MA, Bras A, Medema RH . Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell 2004; 15: 799–811.
Pomerening JR, Ubersax JA, Ferrell JE . Rapid cycling and precocious termination of G1 phase in cells expressing CDK1AF. Mol Biol Cell 2008; 19: 3426–3441.
Enserink JM, Kolodner RD . An overview of Cdk1-controlled targets and processes. Cell Div 2010; 5: 11.
Esashi F, Christ N, Gannon J, Liu Y, Hunt T, Jasin M et al. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 2005; 434: 598–604.
Johnson N, Cai D, Kennedy RD, Pathania S, Arora M, Li YC et al. Cdk1 participates in BRCA1-dependent S phase checkpoint control in response to DNA damage. Mol Cell 2009; 35: 327–339.
Lee SE, Pellicioli A, Demeter J, Vaze MP, Gasch AP, Malkova A et al. Arrest, adaptation, and recovery following a chromosome double-strand break in Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol 2000; 65: 303–314.
Toczyski DP, Galgoczy DJ, Hartwell LH . CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell 1997; 90: 1097–1106.
Yoo HY, Kumagai A, Shevchenko A, Dunphy WG . Adaptation of a DNA replication checkpoint response depends upon inactivation of Claspin by the Polo-like kinase. Cell 2004; 117: 575–588.
Yun MH, Hiom K . CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature 2009; 459: 460–463.
Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K et al. Targets of the cyclin-dependent kinase Cdk1. Nature 2003; 425: 859–864.
Acknowledgements
This work was supported by a NWO-VENI grant (916.76.062) to MATMVV. We thank Drs Maria Jasin, Jeff Parvin, Reuven Agami, James Ferrell Jr and Vera Gorbunova for generously supplying materials, Dr Gerben Vader for critically reading the manuscript and members of the Medical Oncology laboratory for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Krajewska, M., Heijink, A., Bisselink, Y. et al. Forced activation of Cdk1 via wee1 inhibition impairs homologous recombination. Oncogene 32, 3001–3008 (2013). https://doi.org/10.1038/onc.2012.296
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.296
Keywords
This article is cited by
-
Oct4 confers stemness and radioresistance to head and neck squamous cell carcinoma by regulating the homologous recombination factors PSMC3IP and RAD54L
Oncogene (2021)
-
The role of poly(ADP-ribose) polymerase inhibitors in the treatment of cancer and methods to overcome resistance: a review
Cell & Bioscience (2020)
-
Premature activation of Cdk1 leads to mitotic events in S phase and embryonic lethality
Oncogene (2019)
-
Anti-Tumor Effects of Wee1 Kinase Inhibitor with Radiotherapy in Human Cervical Cancer
Scientific Reports (2019)
-
Augmented antitumor activity by olaparib plus AZD1775 in gastric cancer through disrupting DNA damage repair pathways and DNA damage checkpoint
Journal of Experimental & Clinical Cancer Research (2018)