Abstract
Background
It is known that adjuvant chemotherapy improves survival in women with breast cancer. It is not known whether the interval between surgery and the initiation of chemotherapy influences its effectiveness.
Purpose
To determine the relationship between time to initiation of adjuvant chemotherapy and survival in women with breast cancer, through a systematic review of the literature and meta-analysis.
Methods
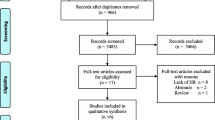
Systematic review of MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Database of Controlled Trials, Google Scholar, and abstracts presented at major international oncology conferences. The primary meta-analysis included only high-validity studies which directly measured the time from surgery to initiation of adjuvant chemotherapy and which controlled for major prognostic factors. Outcomes reported in the original studies were converted to a regression coefficient (β) and standard error corresponding to a 4-week delay in the initiation of chemotherapy. These relative risks were combined in both fixed- and random-effects models. Homogeneity was assessed by the Cochran χ 2 statistic and the I 2 statistic. Potential publication bias was investigated using standard error-based funnel plots.
Results
Meta-analysis of 8 high-validity studies demonstrated that a 4-week increase in TTAC was associated with a significant increase in the risk of death in both the fixed-effects model (RR 1.04; 95 % CI, 1.01–1.08) and random-effects model (RR 1.08; 95 % CI, 1.01–1.15). The association remained significant when the most highly weighted studies were sequentially removed from this analysis, and also when additional, lower validity studies were included in this analysis. Funnel plots showed no significant asymmetry to suggest publication bias.
Conclusions
Increased waiting time from surgery to initiation of adjuvant chemotherapy is associated with a significant decrease in survival. Avoidance of unnecessary delays in the initiation of adjuvant chemotherapy has the potential to save the lives of many women with breast cancer.







Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods, and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Early Breast Cancer Trialists’ Collaborative Group (2012) (EBCTCG). Comparisons between difference polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomized trials. Lancet 379(9814):432–444
National comprehensive care network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer (Version 1. 2016). http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed Mar 23 2016
Ng W, Delaney GP, Jacob S, Barton MB (2010) Estimation of an optimal chemotherapy utilisation rate for breast cancer: setting an evidenced-based benchmark for the best-quality cancer care. Eur J Cancer 46(4):703–712
Colleoni M, Gelber RD (2014) Time to initiation of adjuvant chemotherapy for early breast cancer and outcome: the earlier, the better? J Clin Oncol 32(8):717–719
Brooks RJ, Jones SE, Salmon SE et al (1983) Improved outcome with early treatment in an adjuvant breast program. Proc Am Soc Clin Oncol 2
Buzdar AU, Smith TL, Powell KC, Blumenschein GR, Gehan EA (1982) Effect of timing of initiation of adjuvant chemotherapy on disease-free survival in breast cancer. Breast Cancer Res Treat 2(2):163–169
Cold S, During M, Ewertz M, Knoop A, Moller S (2005) Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG). Br J Cancer 93(6):627–632
Colleoni M, Bonetti M, Coates AS et al (2000) Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The International Breast Cancer Study Group. J Clin Oncol 18(3):584–590
Downing A, Twelves C, Forman D, Lawrence G, Gilthorpe MS (2014) Time to begin adjuvant chemotherapy and survival in breast cancer patients: a retrospective observational study using latent class analysis. Breast J. 20(1):29–36
Farolfi A, Scarpi E, Rocca A et al (2015) Time to initiation of adjuvant chemotherapy in patients with rapidly proliferating early breast cancer. Eur J Cancer 51(14):1874–1881
Gagliato DM, Gonzalez-Angulo AM, Lei X et al (2014) Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32(8):735–744
Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI (2006) Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat 99(3):313–321
Alkis N, Durnali AG, Arslan UY et al (2011) Optimal timing of adjuvant treatment in patients with early breast cancer. Med Oncol 28(4):1255–1259
Kerbrat P, Roche H, Fumoleau P et al (2005) Does time interval between surgery and adjuvant chemotherapy initiation modify treatment efficacy in operable, breast cancer patients? French Adjuvant Study Group (FASG) results. J Clin Oncol 23
Lohrisch C, Paltiel C, Gelmon K et al (2006) Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol 24(30):4888–4894
Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW, Liu CC, Du XL (2013) Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Med Oncol 30(1):419
Pronzato P, Campora E, Amoroso D et al (1989) Impact of administration-related factors on outcome of adjuvant chemotherapy for primary breast cancer. Am J Clin Oncol 12(6):481–485
Ramjeesingh R, Chen BE, Pater JL et al (2015) Association between definitive surgery and times to administration of adjuvant chemotherapy and outcomes in early breast cancer: analysis of adjuvant studies conducted by NCIC clinical trials groups (NCIC CTG). Cancer Res 75
Alliot C (2006) Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Br J Cancer 94(6):938–939
Altundag MK, Celik I, Ozisik Y (2000) Is there a range of time for initiation of adjuvant chemotherapy in patients with malignancy? Ann Oncol 11(9):1209
Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH (2016) Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2(3):322–329
Ghany DA (2013) Impact of adjuvant chemotherapy delay on survival in cancer breast patients. Chinese–German J Clin Oncol. 12(1):20–24
Hamid A, White S (2015) P106 Time to adjuvant chemotherapy in high-risk breast cancer: local outcomes and influences. The Breast. 24(Supplement 1):S62
Vandergrift JL, Breslin TM, Niland JC et al (2012) The association between timing in adjuvant chemotherapy administration and overall survival for women with breast cancer within the National Comprehensive Cancer Network (NCCN). Cancer Res 72(24 Suppl)
JaraSanchez C, Ruiz A, Martin M et al (2007) Influence of timing of initiation of adjuvant chemotherapy over survival in breast cancer: A negative outcome study by the Spanish Breast Cancer Research Group (GEICAM). Breast Cancer Res Treat. 101(2):215–223
Samur M, Bozcuk HS, Dalmaz G et al (2002) Treatment delay in breast cancer; does it really have an impact on prognosis. Turk J Cancer 32(4):138–147
Seneviratne S, Campbell I, Scott N, Kuper-Hommel M, Round G, Lawrenson R (2014) Ethnic differences in timely adjuvant chemotherapy and radiation therapy for breast cancer in New Zealand: a cohort study. BMC Cancer 14:1
Shannon C, Ashley S, Smith IE (2003) Does timing of adjuvant chemotherapy for early breast cancer influence survival? J Clin Oncol 21(20):3792–3797
Trufelli DC, de Matos LL, Santi PX, Del Giglio A (2015) Adjuvant treatment delay in breast cancer patients. Rev Assoc Med Bras 61(5):411–416
Yu KD, Huang S, Zhang JX, Liu GY, Shao ZM (2013) Association between delayed initiation of adjuvant CMF or anthracycline-based chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC Cancer 13:240
Vandergrift JL, Niland JC, Theriault RL et al (2013) Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst 105(2):104–112
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Greenland S (1994) Quality scores are useless and potentially misleading. Am J Epidemiol 140:300–301
Juni P, Witschi A, Bloch R, Egger M (1999) The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 282(11):1054–1060
Stang A (2010) Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P (2014) Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA 312(6):623–630
Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM (2011) Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 305(22):2335–2342
Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ (2008) The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol 87(1):3–16
Schrag D, Cramer LD, Bach PB, Begg CB (2001) Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 93(11):850–857
Savovic J, Jones HE, Altman DG et al (2012) Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 157(6):429–438
Greenland S, O’Rourke K (2008) Meta-analysis. In: Rothman KJ, Greenland S, Lash TL (eds) Modern epidemiology, 3rd edn. Lippincott Williams & Wilkins, Philadelphia
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135(11):1301–1309
Cooper H, Hedges LV, Valentin JC (2009) The handbook of research synthesis and meta-analysis, 2nd edn. Russell Sage Foundation, New York
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Sackett DL (1986) Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 89(2 Suppl):2S–3S
Sackett D, Haynes BR, Guyatt GH, Tugwell P (1991) Deciding whether your treatment has done harm, 2nd edn. Little Brown and Company, Boston
Hill AB (1965) The environment and disease: association or causation? Proc R Soc Med. 58:295–300
Schabel FM (1977) Rationale for adjuvant chemotherapy. Cancer 39(6 Suppl):2875–2882
Schatten WE (1958) An experimental study of postoperative tumor metastases. I. Growth of pulmonary metastases following total removal of primary leg tumor. Cancer 11(3):455–459
Gunduz N, Fisher B, Saffer EA (1979) Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res 39(10):3861–3865
O’Reilly MS, Boehm T, Shing Y et al (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88(2):277–285
O’Reilly MS, Holmgren L, Shing Y et al (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79(2):315–328
Surveillance, epidemiology, and end results (SEER) Program. SEER Stat Fact Sheets: breast cancer. https://seer.cancer.gov/statfacts/html/breast.html. Accessed Mar 23 2016
Funding
Dr Booth holds a Canada Research Chair in Population Cancer Care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
In 2009, Dr Mackillop provided expert testimony on the relationship between delays in post-lumpectomy radiotherapy for breast cancer and the risk of local recurrence, in a class action lawsuit in Quebec (Cilinger v Centre Hospitalier de Chicoutimi). No other disclosures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raphael, M.J., Biagi, J.J., Kong, W. et al. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 160, 17–28 (2016). https://doi.org/10.1007/s10549-016-3960-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3960-3