Abstract
Objective
Effective cytotoxic treatment options for advanced cervical cancer are exceedingly limited. Therefore, interest has increased in targeted therapeutics for the treatment of cervical cancer. Cetuximab, a monoclonal antibody, binds specifically to the epidermal growth factor receptor (EGFR) and competitively inhibits the binding of epidermal growth factor and other ligands. In cervical cancer the expression of EGFR is reported in up to 85% of the tumour cells. Therefore, Cetuximab monotherapy could be a new option in the treatment of patients with advanced cervical cancer.
Study design
Five patients with advanced cervical cancer were treated with Cetuximab monotherapy as third- to fifthline therapy between 2005 and 2008 in our institution. The tumour stage at the time of diagnosis ranged between IIB and IVB. Cetuximab was applied with an initial loading dose of 400 mg/m2 followed by a dose of 250 mg/m2 weekly.
Results
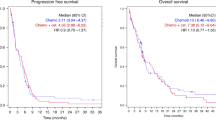
Only one patient (20%) had a stable disease, and the other four a progressive disease during Cetuximab monotherapy, after the RECIST criterias. Four out of five patients (80%) developed an acneiform rash as a common observed side effect of Cetuximab therapy. The median survival time from the beginning of the Cetuximab therapy was 8.6 months.
Conclusion
No advantage could be found in the treatment with Cetuximab monotherapy in patients with advanced cervical cancer in this study. Further studies are necessary to evaluate the significance of Cetuximab in the treatment of advanced cervical cancer.
Similar content being viewed by others
References
Bonomi P, Blessing JA, Stehman FB, DiSaia PJ, Walton L, Major FJ (1985) Randomized trial of three cisplatin dose schedules in squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 3(8):1079–1085
Rose PG, Blessing JA, Gershenson DM, McGehee R (1999) Paclitaxel and cisplatin as firstline therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 17(9):2676–2680
Burnett AF, Roman LD, Garcia AA, Muderspach LI, Brader KR, Morrow CP (2000) A phase II study of gemcitabine and cisplatin in patients with advanced, persistent, or recurrent squamous cell carcinoma of the cervix. Gynecol Oncol 76(1):63–66
Long HJ 3rd, Bundy BN, Grendys EC Jr et al (2005) Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a gynecologic oncology group study. J Clin Oncol 23(21):4626–4633
Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J (1995) Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumour xenograft model. Clin Cancer Res 1(11):1311–1318
Bonner JA, Harari PM, Giralt J et al (2006) Radiotherapy plus cetuximab for squamous cell carcinoma of the head and neck. N Engl J Med 354(6):567–578
Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22(7):1201–1208
Vermorken JB, Trigo J, Hitt R et al (2007) Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 25(16):2171–2177
Bellone S, Frera G, Landolfi G et al (2007) Overexpression of epidermal growth factor type-1 receptor (EGF-R1) in cervical cancer: implications for Cetuximab-mediated therapy in recurrent/metastatic disease. Gynecol Oncol 106(3):513–520
Interdisziplinäre Leitlinie der Deutschen Krebsgesellschaft e.V. (DKG) und der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG); AGO Kommission Uterus Diagnostik und Therapie des Zervixkarzinoms DKG 2008:032/033 (S2 k)
Alvarez G, Perry A, Tan BR, Wang HL (2006) Expression of epidermal growth factor receptor in squamous cell carcinomas of the anal canal is independent of gene amplification. Mod Pathol 19(7):942–949
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumours. J Natl Cancer Inst 92(3):205–216
Pfeiffer D, Stellwag B, Pfeiffer A, Borlinghaus P, Meier W, Scheidel P (1989) Clinical implications of the epidermal growth factor receptor in the squamous cell carcinoma of the uterine cervix. Gynecol Oncol 33(2):146–150
Gullick WJ, Marsden JJ, Whittle N, Ward B, Bobrow L, Waterfield MD (1986) Expression of epidermal growth factor receptors on human cervical, ovarian, and vulval carcinomas. Cancer Res 46(1):285–292
Kurtz JE, Hardy-Bessard AC, Deslandres M et al (2009) Cetuximab, topotecan and cisplatin for the treatment of advanced cervical cancer: a phase II GINECO trial. Gynecol Oncol 113(1):16–20
Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD (2009) Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 27(7):1069–1074
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hertlein, L., Lenhard, M., Kirschenhofer, A. et al. Cetuximab monotherapy in advanced cervical cancer: a retrospective study with five patients. Arch Gynecol Obstet 283, 109–113 (2011). https://doi.org/10.1007/s00404-010-1389-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1389-1