Article Text
Abstract
Objective To evaluate the added value of a centralized pathology review of the diagnoses of gestational trophoblastic diseases by expert pathologists and its potential impact on clinical management in a prospective multicenter study based on the Belgian Gestational Trophoblastic Diseases Registry.
Methods From July 2012 to December 2020, the two referral centers of the registry were solicited to advise on 1119 cases. Referral pathologists systematically reviewed all of the initial histological diagnoses. Cases initially assessed by expert pathologists were excluded. A total of 867 files were eligible for the study. Concordance between diagnoses of gestational trophoblastic diseases made by general ‘non-expert’ and expert pathologists was analyzed together with the potential impact of the alterations on clinical management. Expert pathologists were working in an academic setting with high exposure to placental pathology and national recognition.
Results The rate of discordance between expert and non-expert pathologists for the initial diagnoses was 35%. Almost 95% of complete moles were confirmed by the expert pathologists, but only 61% for partial moles. Compared with previous studies, ancillary techniques (p57 immunohistochemistry, karyotype) were used twice as often by both groups of pathologists in this survey. The diagnosis of gestational trophoblastic neoplasia was altered in 42% of cases. When the initial diagnosis was altered, the clinical relevance of this correction was estimated as down staging, up staging, or not relevant in 65%, 33% and 2% of cases respectively.
Conclusion Systematic centralized pathological review of gestational trophoblastic diseases modified the diagnosis in a third of cases. The results also show that a change in diagnosis would impact clinical management in 98% of patients.
- Gestational Trophoblastic Disease
- Hydatidiform Mole
- Trophoblastic Neoplasms
- Pathology
Data availability statement
Data are available upon reasonable request. Data are filed by the first author and are available on request.
Statistics from Altmetric.com
HIGHLIGHTS
Centralized histological reviews are beneficial for the diagnosis of rare pathologies.
In the Belgian Gestational Trophoblastic Diseases Registry, systematic pathological review by expert pathologists modified 35% of the initial diagnoses.
The diagnostic alteration impacted clinical management in the majority of cases.
Introduction
Gestational trophoblastic diseases include a spectrum of rare placental pathologies, ranging from benign fertilization anomalies (hydatidiform moles) to malignant lesions, termed gestational trophoblastic neoplasia, which encompass invasive mole, gestational choriocarcinoma, placental site trophoblastic tumor, and epithelioid trophoblastic tumor.1 Placental proliferative disorders can also lead to tumor-like lesions (exaggerated placental site reaction and placental site nodule). Molar pregnancies have an incidence rate ranging from 1/1000 to 2/1000 pregnancies in Europe and Asia, respectively.2 Most cases are sporadic, but familial recurrent hydatidiform moles have been reported.3 4 Among complete moles, 15–20% transform into post-molar gestational trophoblastic neoplasia with persistently elevated human chorionic gonadotropin levels, of which 3% are choriocarcinomas. Up to 3% of partial moles progress to post-molar gestational trophoblastic neoplasia, of which 0.5% are choriocarcinomas.5 The incidence of choriocarcinoma is estimated to be 1–9 per 40 000 pregnancies.6 Due to their rarity and heterogeneity, the differential diagnosis between these entities is often challenging. Because their clinical management and prognosis can differ significantly, a precise initial diagnosis is important.
Gestational trophoblastic neoplasia may be cured in 98% of cases if a correct diagnosis is made initially.7 Choriocarcinoma is monitored by serum levels of human chorionic gonadotropin and responds well to chemotherapy, while placental site trophoblastic and epithelioid trophoblastic tumors are associated with low levels of human chorionic gonadotropin and are more chemoresistant, requiring hysterectomy if confined to the uterus.8
A diagnosis of gestational trophoblastic disease is based on histopathology. Ultrasound has a low sensitivity of 34%, hence the importance of histological examination of curettage products.9 Despite established histological criteria, many cases are confounding, leading to an overdiagnosis of hydatidiform moles. In extrauterine pregnancies and early spontaneous abortions, hydropic villous trophoblastic tissue may suggest a partial mole.10 Morphologic examination has interobserver variability, emphasizing the need for ancillary techniques to refine diagnosis.11
Advanced anatomopathological or genetic methods (immunohistochemistry, fluorescence in situ hybridization, karyotype, and genotyping) can lead to a more accurate diagnosis. Immunohistochemistry of antigen p57 is useful to distinguish complete from partial moles. CDKN1C, the gene coding for p57, undergoes paternal imprinting. Its expression exclusively depends on maternal chromosomes. p57 is thus missing in stromal villous cells and villous cytotrophoblasts of complete moles. However, p57 cannot differentiate partial mole from non-molar hydropic abortion, since they both show p57 immunolabelling. Hydatidiform moles have specific genetic features: androgenetic diploidy for complete moles and diandric triploidy for partial moles. When a partial mole is suspected, ploidy analysis is helpful to exclude diploid hydropic abortion. Staining for the nuclear proliferation marker Ki67 can help to differentiate benign tumor-like lesions from gestational trophoblastic neoplasia.
In 1971, Brewer et al were the first to highlight the benefit of gestational trophoblastic diseases management by experienced teams in terms of treatment morbidity and mortality.12 Registration systems were since adopted and reference centers were progressively established in European countries (UK in 1972, Netherlands in 1977, France in 2000, Switzerland in 2009, and Belgium in 2012) and worldwide. The benefits of expert pathological reviews are well documented in the literature. The agreement rate between ‘non-expert’ and expert pathologists has been reported to be 50–64% and 96% for partial and complete moles, respectively.13 14 It is not clear, however, what the impact is on clinical management. In Belgium, the Belgian Cancer Registry recorded only epidemiological data on choriocarcinomas15 16 until the establishment of the Belgian Gestational Trophoblastic Registry in 2012.
Methods
A prospective multicenter study on the impact of a centralized pathological review of histological diagnoses of gestational trophoblastic diseases was conducted. In 2012, under the aegis of the Belgian and Luxembourg Gynecological Oncology Group, the Belgian Gestational Trophoblastic Registry was started, and two reference centers (a French speaking and a Flemish speaking) were established, with support from Groupement des Gynécologues Obstétriciens de la Langue Française, Vlaamse Vereniging voor Obstetrie en Gynaecologie, and European Organization for the Treatment of Trophoblastic Diseases.17 Cases referred to the two centers were collected between July 2012 and December 2020. The objectives of the centers were to record and review diagnoses of gestational trophoblastic diseases, but also to provide clinical advice and assistance with follow-up.18 Cases were referred on a voluntary basis, by the initial pathologist, gynecologist, or the patient. Each initial histological diagnosis was systematically reviewed by at least one expert pathologist, defined as a pathologist working in an academic setting with high exposure to placental pathology and national recognition. Histological slides were requested from the local pathology laboratories and were reviewed by referral pathologists from the University Hospitals of Liège, Saint-Luc, Erasmus, and Leuven. Cases referred for clinical advice without pathological material available, cases with insufficient histological material, and cases without diagnostic review were excluded (n=85).
Cases were classified according to the 2020 World Health Organization classification19 as: non-molar pregnancy, exaggerated placental site reaction, placental site nodule and plaque with or without atypia, partial hydatidiform mole, complete hydatidiform mole, invasive mole, gestational choriocarcinoma, placental site trophoblastic tumor, epithelioid trophoblastic tumor, not specified (if the initial diagnosis was not mentioned), mole not otherwise specified (if hydatidiform mole was suspected without a precise diagnosis), and gestational trophoblastic neoplasia not otherwise specified (if trophoblastic neoplasia was suspected without a precise diagnosis).
To accurately assess concordance between the initial diagnoses of ‘non-expert’ and expert pathologists, cases referred originally to the experts were not taken into account (n=167). The clinical relevance of a change between the initial and revised diagnosis was evaluated. Discordances were classified as up staging if the pathology review resulted in a more severe diagnosis or in a more intensive management, as down staging if the review resulted in a less severe diagnosis or in a less intensive management, and as not relevant if the review did not modify the therapeutic management. For patients referred to the centers with no mention of the initial diagnosis, we noticed that gestational trophoblastic disease was suspected, but most often not specified. Therefore, the clinical impact was considered as down staging if the reviewed diagnosis was non-molar pregnancy. In case of mole not otherwise specified, we considered that the clinical impact of the rereading was up staging in the case of complete mole and down staging in the case of partial mole. When the initial diagnosis of placental site trophoblastic tumor or epithelioid trophoblastic tumor was changed to choriocarcinoma or invasive mole, the clinical impact was considered as down staging because placental site trophoblastic tumor and epithelioid trophoblastic tumor are rare entities for which the prognosis is worse and management is more complicated.
Histological–morphological analysis was performed on hematoxylin–eosin slides. Ancillary techniques were performed when needed, mainly p57 immunohistochemistry, to confirm complete moles. We were not able to identify whether the diagnostic revisions by the panel of experts was based only on the morphological analysis or also on ancillary tests.
We analyzed the level of concordance between the French speaking center’s expert pathologists: fully concordant when all experts agreed on the diagnosis, partially concordant when one of the experts did not agree on the diagnosis, and fully discordant when each experts submitted a different diagnosis.
Results
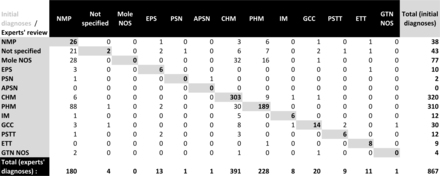
A total of 1119 cases were submitted to the centers (835 for the French speaking part, 284 for the Flemish speaking part). The activity of the centers has steadily grown from an annual recruitment of 81 cases in 2013 to 179 in 2020. Eighty-five files were excluded because of missing data. Among the 1034 selected files, 167 cases (16%) were excluded because they were primarily assessed by an expert pathologist. Finally, 867 files were eligible for analysis (Figure 1). The global alteration rate of the initial ‘non-expert diagnoses by expert pathologists was 35%, and was 69% in cases of ectopic pregnancy (Figure 2). The clinical relevance of this revision was down staging in 65% of cases, up staging in 32%, and not relevant in 2% (Figure 3). The reviewed diagnoses were 93% fully concordant, 5% partially concordant, and 2% fully discordant between expert pathologists.
Flowchart describing the selection procedure of the cases eligible for the study.
Initial diagnoses by local pathologists versus expert pathologists. Gray cases=concordance between initial diagnoses and expert diagnoses. NMP, non-molar pregnancy; Mole NOS, mole not otherwise specified; EPS, exaggerated placental site; PSN, placental site nodule without atypia; APSN, placental site nodule and plaque with atypia; CHM, complete hydatidiform mole; PHM, partial hydatidiform mole; IM, invasive hydatidiform mole; GCC, gestational choriocarcinoma; PSTT, placental site trophoblastic tumor; ETT, epithelioid trophoblastic tumor; GTN NOS, gestational trophoblastic neoplasia not otherwise specified.
Modification rate of the initial diagnoses by the expert review and its clinical relevance for each diagnostic category. Up staging=if the pathology review resulted in a more severe diagnosis or in a more intensive management. Down staging=if the review resulted in a less severe diagnosis or in a less intensive management. Not relevant=if the review did not modify the therapeutic management. NMP, non-molar pregnancy; Mole NOS, mole not otherwise specified; EPS, exaggerated placental site; PSN, placental site nodule without atypia; CHM, complete hydatidiform mole; PHM, partial hydatidiform mole; IM, invasive hydatidiform mole; GCC, gestational choriocarcinoma; PSTT, placental site trophoblastic tumor; ETT, epithelioid trophoblastic tumor; GTN NOS, gestational trophoblastic neoplasia not otherwise specified.
Review of Hydatidiform Moles
A total of 832 cases of hydatidiform moles were referred to the registry, of which 125 were excluded because they were submitted by expert pathologists. The rate of agreement for hydatidiform moles after the reviewing process was 82%. p57 labeling was performed in 61% of cases and reached 96% when a complete mole was suspected initially.
A total of 363 partial moles were referred (35%), of which 53 cases were not retained because they were submitted by expert pathologists. The rate of diagnostic alteration by the reviewing was 38%. Three-quarters of the disagreements were altered as non-molar pregnancy. The remaining were reclassified as complete moles in 25% and exaggerated placental site in 2% (Figure 4A). Karyotype was available in 10% of the cases.
Results of the review of initial diagnoses of partial moles–complete moles–gestational trophoblastic neoplasia by the expert pathologists. Pie charts showing the results of the diagnostic review by expert pathologists for partial moles (A), complete moles (B), and gestational trophoblastic neoplasia (C). Percentage of confirmed diagnoses (black) and percentage of diagnostic changes (gray). NMP, non-molar pregnancy; EPS, exaggerated placental site; CHM, complete hydatidiform mole; PHM, partial hydatidiform mole; IM, invasive hydatidiform mole; GCC, gestational choriocarcinoma; PSTT, placental site trophoblastic tumor; ETT, epithelioid trophoblastic tumor; GTN NOS, gestational trophoblastic neoplasia not otherwise specified.
Three hundred and ninety complete moles were submitted to the centers, of which 70 cases were excluded because they were referred by expert pathologists. The alteration rate was 5%: nine cases were reclassified as partial moles (53%), six as non-molar pregnancies (35%), one as invasive mole (6%), and one as choriocarcinoma (6%) (Figure 4B). When a hydatidiform mole was initially suspected, not otherwise specified, the reviewers diagnosed a complete mole in 42%, a non-molar pregnancy in 36%, and a partial mole in 21% of cases. One case was altered as choriocarcinoma.
For the not specified initial diagnoses, the diagnoses of the expert pathologists were distributed as follows: 49% non-molar pregnancies, 16% partial moles, 14% complete moles, 7% tumor-like lesions, and 9% gestational trophoblastic neoplasia. After the centralized review, hydatidiform moles accounted for 71% of the final diagnoses of the registry. Complete moles represented the majority of cases submitted to the centers: 45% versus 27% for partial moles. The mean age of patients was 31 years and 33 years for partial (14–42 years) and complete (13–55 years) respectively.
Review of Gestational Trophoblastic Neoplasia
Eighty-seven cases of gestational trophoblastic neoplasia were submitted to the centers, of which 20 were excluded because they were initially diagnosed by expert pathologists. The overall rate of agreement after the review was 58%. Cases with disagreement were reviewed as complete moles in 61%, non-molar pregnancies in 25%, exaggerated placental site reactions in 7%, and partial moles in 4% of cases (Figure 4C). Expert pathologists confirmed the diagnosis of choriocarcinoma in 47% of cases, while 27% were reclassified as complete moles and three cases as non-molar pregnancies. The four remaining cases were diagnosed as invasive mole, placental site trophoblastic tumor, and gestational trophoblastic neoplasia not otherwise specified.
The concordance rate was 50% and 89% in the case of initial diagnosis of invasive mole/placental site trophoblastic tumor and epithelioid trophoblastic tumor, respectively. After the centralized review, gestational trophoblastic neoplasia represented 6% of the final diagnoses, experts included. Mean age of patients was 38 years (19–60 years).
Discussion
Summary of Main Results
The global diagnostic alteration rate was 35%. The global rate of agreement for hydatidiform moles was 82%. Non-molar pregnancy was the final diagnosis in 21% of cases, of which 50% were initially diagnosed as partial moles. When non-expert pathologists suspected a hydatidiform mole not otherwise specified, the experts always concluded with a specific diagnosis, including one gestational trophoblastic neoplasia. The reviewers agreed with 58% of the initial diagnoses of gestational trophoblastic neoplasia. Only 47% of the initial diagnoses choriocarcinoma were confirmed.
The concordance rate between the expert pathologists of the three French speaking university departments was 93%; it was not calculated for the Flemish speaking experts because all of the cases from this center were analyzed in one department (KU Leuven). Given the high concordance rate between expert pathologists, initial diagnoses performed by them were excluded from the analysis to avoid underestimation of diagnostic alterations.
Results in the Context of Published Literature
The benefit of centralized histological reviews of neoplasia by experienced teams has been highlighted previously. This strategy is applied in malignant pathologies, such as thymic epithelial tumors,20 sarcomas,21 thyroid cancer,22 melanocytic lesions,23 and mesothelioma.24 Overall, the rate of diagnostic revisions by pathology reviews ranged from 5% to 40%. Consequently, the European Organization for the Treatment of Trophoblastic Diseases has encouraged the formation of gestational trophoblastic disease reference centers and proposed clinical guidelines to standardize their management.25 Despite pathologists’ awareness about the diagnostic complexity and rarity of these diseases, this alteration rate has been stable over the past 20 years.13 14 This may be due to the low incidence of the pathology and thus a low exposure of non-expert pathologists. Indeed, given the number of pathologists in Belgium, each is exposed to approximately only one case every 3 years.26
Expert pathologists confirmed only 62% of partial moles, which concords with previous published rates (50%,13 64%14). The level of agreement for complete moles was 95%, a high concordance rate that is particularly stable in the literature (96% in 199813 and 201114). The p57 analysis was used in 90% of suspected complete moles (51% in 201114). The addition of this ancillary technique enhanced the diagnostic accuracy by 20%.11 27 Our results confirm that hydatidiform moles are over diagnosed in ectopic pregnancies.28
We identified less partial (276/738) than complete moles (462/738), in contrast with the literature showing a threefold higher incidence of partial moles.1 A possible explanation could be that partial moles are less frequently referred to the centers because they are considered less dangerous. This represents referral bias, which may underestimate the impact of centralized rereading, given that the rate of diagnostic alteration by expert pathologists is high when a partial mole is suspected initially. The agreement rate was slightly lower in our results for gestational trophoblastic neoplasia (58% vs 71% in 201114).
Strengths and Weaknesses
The strengths of our study are the prospective and multicenter design of the registry. The pathological alterations were carried out by expert Belgian pathologists. Moreover, p57 labeling was available for the majority of complete moles. Compared with previous studies,13 14 we showed that the proportion of revised diagnoses remained stable despite the larger application of ancillary techniques and that the diagnostic alteration would impact clinical management in 98% of patients.
We acknowledge some limitations of this study. First, because registration was on a voluntary basis, a proportion of cases may have been missed. However, the number of cases we recruited was comparable with that expected from the available epidemiological data. We diagnosed 142 gestational trophoblastic diseases in 2020, while the birth rate in Belgium was 113 739,29 representing an estimated incidence rate of 1.25 per 1000 pregnancies. Second, the pathological material was sent for review, often without the possibility of performing genetic analysis. For example, analysis of the ploidy would have been helpful to confirm the diagnosis of partial moles in the case of triploidy. Genotyping would have been of great interest for the diagnosis of trophoblastic neoplasia by detecting the presence of the paternal genome.
Implications for Practice and Future Research
Our results showed that a third of patients would have received an inaccurate management without the review. After diagnostic alteration, 65% of diagnoses were down staged. Without the review, patients would have received more intensive treatment or follow-up, causing more anxiety and side effects. In contrast, 32% of the diagnoses were up staged, meaning that patients would have been undermanaged, probably resulting in a higher risk of unfavorable outcomes. Only 2% of the modifications had no clinical impact.
Partial moles have a confounding morphological aspect with non-molar hydropic miscarriages, particularly early in the first trimester, hence the utility of a genetic analysis to exclude a biparental diploid miscarriage mimicking a diandric triploid partial mole.30 Unfortunately, a karyotype is rarely available, probably because this analysis requires fresh tissue, which is not always available.
In case of a choriocarcinoma reviewed as a placental site trophoblastic tumor, the patient would have received polychemotherapy while the recommended treatment is surgery. Three patients would have received unnecessary chemotherapy for a miscarriage. Eight cases of presumed choriocarcinoma were reviewed as complete moles, which only require a wait and see approach with regular monitoring of human chorionic gonadotropin levels. Half of the initial placental site trophoblastic tumor diagnoses were modified to benign trophoblastic diseases by the review, avoiding unnecessary hysterectomies in these patients.
Conclusion
This study showed that a third of diagnoses of gestational trophoblastic diseases were modified after systematic revision by expert pathologists. This highlights the importance of centralization of all diagnoses to referral pathologists. Despite the pathologists’ awareness of the diagnostic challenge of this pathology, this rate has been remarkably consistent over the past decades. These results support the European Organization of Trophoblastic Diseases’ recommendation to create reference centers in European countries.
Data availability statement
Data are available upon reasonable request. Data are filed by the first author and are available on request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the medical ethics committee of the University Hospitals-University of Leuven, Belgium (No B322201214659S S53375). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We thank the Belgian and Luxembourg Gynecological Oncology Group, Groupement des Gynécologues Obstétriciens de la Langue Française, and the Vlaamse Vereniging voor Obstetrie en Gynaecologie, for hosting the registry and for their support. We also acknowledge the patients, the data nurses of the two centers for collecting patient records and acquiring the data, and our colleagues.
References
Footnotes
Contributors FG and SH established the study design, participated in patient recruitment and data analysis, and revised and approved the final manuscript. SS participated in data acquisition and analysis, performed data and statistical analyses, and wrote the manuscript. KD, A-SVR, EM, J-CN, PD, and PM participated in patient recruitment and pathological review, and revised and approved the final manuscript. IV and FK contributed to the establishment of the study design, and revised and approved the final manuscript. AV participated in the data acquisition. FG is the guarantor of this study.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.