Article Text
Abstract
Objective The association of primary oncologist specialty, medical oncology versus gynecologic oncology, on intensity of care at the end of life in elderly patients with gynecologic cancer is unclear.
Methods This retrospective cohort study used Surveillance, Epidemiology and End Results-Medicare (SEER-M) data. Subjects were fee-for-service Medicare enrollees aged 65 years and older who died of a gynecologic cancer between January 2006 and December 2015. The primary outcome was a composite score for high-intensity care received in the last month of life. Secondary outcomes included invasive procedures and Medicare spending in the last month of life. Simple and multivariable linear and logistic regression analyses evaluated differences in outcomes by primary oncologist specialty. Linear regressions were repeated after creating a more similar control group through nearest-neighbor propensity score matching.
Results Of 12 189 patients, 7705 (63%) had a medical primary oncologist in the last year of life. In adjusted analyses, patients with a gynecologic versus medical primary oncologist received lower rates of high-intensity end-of-life care (53.9% vs 56.6%; p=0.018). Results were similar for the propensity score-matched cohorts. However, having a gynecologic versus medical primary oncologist was associated with higher rates of invasive procedures in the last month of life (43% vs 41%; p=0.014) and higher Medicare spending ($83 859 vs $74 849; p=0.004).
Conclusions Both specialties engage in overall high levels of intense end-of-life care, with differences by specialty in aspects of aggressive care and spending at the end of life. Physician-level training could be a target for educational or quality improvement initiatives to improve end-of-life cancer care delivery.
- Gynecology
- Quality of Life (PRO)/Palliative Care
Data availability statement
Data may be obtained from a third party and are not publicly available.
Statistics from Altmetric.com
HIGHLIGHTS
Having a gynecologic rather than a medical oncologist was associated with lower rates of high-intensity end-of-life care.
Having a gynecologic oncologist rather than a medical oncologist was associated with higher rates of invasive procedures.
Having a gynecologic oncologist rather than a medical oncologist was associated with higher Medicare spending.
Introduction
Aggressive medical end-of-life care has been associated with worse quality of life in patients with advanced cancer and worse adjustment for their bereaved caregivers.1 Older women with ovarian cancer increasingly experience high-intensity medical care in the last year of life, despite increasing rates of hospice utilization.2 These trends are consistent with evidence that healthcare utilization by gynecologic cancer patients increases significantly in the last 2 months of life.3 Subsequent high healthcare costs are associated with a worse quality of life immediately prior to death, and have been shown to be decreased by end-of-life discussions.4
Understanding the role of the primary oncologist at the end of life is imperative because the majority of gynecologic cancer patients are fully managed by their primary oncology provider, with only a minority cared for by palliative care specialists.5 6 Gynecologic oncology patients may get their care with either a gynecologic oncologist, who can provide both surgical and medical treatments, or a medical oncologist, who provides only medical treatments. In addition to their medical training, medical and gynecologic oncologists differ in their scope of practice, degree of specialization, and practice structures, all of which may influence their approach to end-of-life care. A prior study found that primary oncology provider specialty does not impact cancer survival outcomes, although gynecologic oncologists provide overall less aggressive chemotherapy treatment.7 A recent study evaluating physician influence on aggressive end-of-life care among women who died of ovarian cancer found that physician specialty was associated with differences in hospice enrollment, chemotherapy, and life-extending procedures.8 However, the study did not examine other gynecologic cancer sites and did not directly compare medical oncologists with gynecologic oncologists.
A greater understanding of the impact of primary oncologist specialty on end-of-life care is important as gynecologic oncology continues to develop collaborative models of care and works to improve palliative care and end-of-life training.9 10 The objectives of this study were to examine the association of primary oncologist specialty on intensity of care and Medicare spending with end of life among gynecologic cancer patients.
Methods
Data
This retrospective cohort study used Surveillance, Epidemiology and End Results-Medicare (SEER-M) data, which are drawn from a nationally representative sample of United States cancer patients and are linked with Medicare claims data.11 In accordance with the journal’s guidelines, we will provide our data for the reproducibility of this study in other centers if such is requested.
Study Population
Our cohort included female patients who died of a gynecologic cancer (ovary, uterus, cervix, vagina, vulva, or other female genital site) between January 2006 to December 2015, were enrolled in Medicare Parts A and B for the last 12 months of life, and did not have end-stage renal disease. We excluded patients who had more than one gynecologic cancer, a cancer that was diagnosed on autopsy, inconsistent SEER and Medicare dates of death, or zero outpatient visits with a gynecologic or medical oncologist in the last year of life (Online supplemental figure 1). In addition to this full cohort sample, we created a smaller sample of propensity score-matched patients (Online supplemental file).
Supplemental material
Supplemental material
Exposure
Medical and gynecologic oncologists were identified using the specialty listed in physician claims. Gynecologic oncologists were defined as any providers who had any specialty codes of “Gynecological/Oncology” (specialty code 98). Medical oncologists were defined as any providers who had any specialty codes of “Medical Oncology” (specialty code 90) or “Hematology/Oncology” (specialty code 83). This variable has been shown to accurately identify physician specialty in over 80% of oncologists.12 13
Each patient was assigned to a primary medical oncologist or a primary gynecologic oncologist based on the specialty of their primary oncologist, defined as the provider with the plurality of outpatient oncology visits in the last year of life. A similar algorithm was found to correctly attribute approximately 85% of patients to their anti-cancer treatment-prescribing oncologist.14 Outpatient visits were identified based on Healthcare Common Procedure Coding System (HCPCS) codes (Online supplemental table 4). Ties were broken by assigning the patient to the last outpatient provider seen before death. See Online supplemental file for details on additional sensitivity analyses.
Primary Outcome
The primary outcome, high-intensity end-of-life care, was a binary composite score of intense care in the last 30 days of life. The score has been used in prior literature examining end-of-life care in patients with cancer.15 16 The composite score was defined by any of the following: receipt of chemotherapy in the last 14 days of life,17 18 death in the hospital,17–19 enrollment in hospice for less than 3 days,17 18 more than one emergency department visit in the last 30 days of life,17–20 more than one hospital admission in the last 30 days of life,17 18 20 spending more than 14 days in the hospital in the last 30 days of life,3 17 or any intensive care unit (ICU) admission in the last 30 days of life.17–20 We also examined each individual component of the composite score.
Secondary Outcomes
The secondary outcomes included invasive procedures in the last 30 days of life3 and Medicare spending in the last 30 days of life. Invasive procedures were defined as any invasive procedure, test, or part of care that involved some amount of pain or discomfort. Over 1200 HCPCS and International Classification of Diseases (ICD) codes were manually selected. Invasive procedure categories included surgery, biopsies, cardiac catheterization and procedures, central lines, cardiopulmonary resuscitation, dialysis, drain insertion or exchange, upper and lower endoscopy, gastrostomy tube placement or exchange, incision and drainage, intubation, interventional radiology procedures, total parenteral nutrition, tracheostomy, indwelling bladder catheterization, mechanical ventilation, and wound care. Non-invasive tests, venipuncture, injections, and medications were excluded.
Medicare spending was defined as the total allowed Medicare payment amount, a sum of Medicare spending in the last 30 days of life for inpatient and outpatient, physician/suppliers, home health agency, hospice, durable medical equipment, and Medicare Part D claims. Suppliers includes non-physician providers including advanced practice providers, social workers, some laboratories, emergency medical services providers, and some ambulatory surgical centers. Claims within 30 days after the date of death were included to allow for a lagged claims submission.
Statistical Analysis
We used linear regression models to estimate the association between primary oncologist specialty and the intense end-of-life binary composite score. Simple (bivariate) and multivariable linear regressions were conducted for each of the three cohorts: (1) entire study population; (2) propensity score-matched with replacement; and (3) propensity score-matched without replacement. The multivariable linear regression for the entire study population included covariates for age at death, race, ethnicity, marital status, median income of residential zip code, percent of people with less than a high school education in the residential zip code, SEER registry source, residential urban status, year of diagnosis, year of death, cancer site, cause of death, stage at diagnosis, Medicare/Medicaid dual eligibility, and Charlson Comorbidity Index (CCI) at death. The multivariable linear regression for the propensity score-matched cohorts included only unbalanced covariates. We repeated these analyses with logistic rather than linear regressions.
Similar linear regression models tested the association between specialty of the primary oncologist and other outcomes: each component of the composite score, the secondary outcomes listed earlier, and the composite score by cancer site. Standard errors for all models were clustered at the primary oncologist level. All statistical tests were considered significant at p<0.05. All analyses were performed with Stata 15.0.21 This study was reviewed by the University of Pennsylvania institutional review board and was determined to be exempt.
Results
Cohort
A total of 22 554 women who met the following initial criteria were assessed for eligibility. The final cohort included 12 189 women. Overall, most of the cohort was aged less than 80 years (61%), White (>80%), and died of ovarian cancer (55%). Medical oncologists served as the primary oncologist for the majority of patients (n=7705, 63%) in the last year of life. Table 1 lists the patient characteristics.
Patient characteristics
High-Intensity End-of-Life Care
Overall, the majority of women in the cohort received high-intensity end-of-life care (n=6778, 56%). In unadjusted analyses, patients with a primary medical oncologist were slightly more likely to receive high-intensity end-of-life care (n=4354, 57%) compared with patients with a primary gynecologic oncologist (n=2424, 54%; p=0.028). A similar difference was seen in adjusted analyses (57% vs 54%; p=0.018) with gynecologic oncologists’ patients having lower odds of receiving high-intensity end-of-life care (odds ratio (OR) 0.90, p=0.018). Similar results were seen in the propensity score-matched cohorts (Table 2).
Difference in composite rate of high-intensity end-of-life care by primary outpatient oncologist type
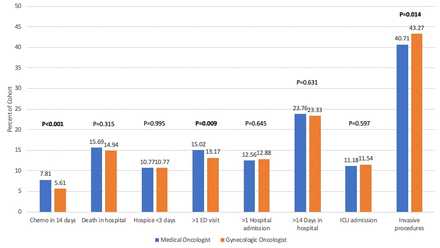
Most patients who received high-intensity end-of-life care received only one measure of high-intensity care (Online supplemental figure 2). Figure 1 and Online supplemental table 5 show the components of the high-intensity end-of-life care composite score. Overall, 7% (n=855) received chemotherapy in the last 14 days of life, 15% (n=1879) died in the hospital, 11% (n=1313) were enrolled in hospice for less than 3 days, 14% (n=1748) had more than one emergency department visit, 13% (n=1545) had more than one hospital admission, 24% (n=2877) spent more than 14 days in the hospital, and 11% (n=1379) had an ICU admission in the last 30 days of life. In adjusted analyses, patients with a primary medical oncologist were more likely to receive chemotherapy in the last 14 days of life (8% vs 6%; p<0.001) and have more than one emergency department visit (15% vs 13%; p=0.009). Ovarian cancer was the only cancer site associated with a statistically significant difference in high-intensity end-of-life care by primary oncologist type (56% vs 53%; p=0.038), with gynecologic oncologists’ patients having lower odds of receiving high-intensity end-of-life care (OR 0.89, p=0.044; Online supplemental table 6).
Supplemental material
End-of-life care outcomes by primary oncologist specialty. ED, emergency department; ICU intensive care unit.
Invasive Procedures
Overall, 42% (n=5077) of women in the cohort received an invasive procedure in the last 30 days of life. In unadjusted analyses, there was not a significant difference in rates of invasive procedures between medical oncologists’ patients (n=3155, 41%) compared with gynecologic oncologists’ patients (n=1922, 43%; p=0.068). However, in adjusted analyses (Figure 1 and Online supplemental table 5), gynecologic oncologists’ patients were more likely to undergo an invasive procedure (43%) compared with medical oncologists’ patients (41%; p=0.014). The 25 most common procedure codes in the last 30 days of life are presented in Online supplemental table 7.
Medicare Spending
The mean Medicare spending in the last 30 days of life for the entire cohort was $76 776. In unadjusted analyses, there was not a significant difference in Medicare spending in the last 30 days of life between medical oncologists’ patients ($76 776) compared with gynecologic oncologists’ patients ($80 548; p=0.263). However, in adjusted analyses, care with gynecologic oncologists was associated with significantly higher spending compared with care with medical oncologists ($83 859 vs $74 849; p=0.004). Similar results were seen in the propensity score-matched cohorts (Table 3).
Difference in overall Medicare spending in the last 30 days of life by primary outpatient oncologist type
Table 4 shows Medicare spending in the last 30 days of life by claim type. In adjusted analyses, care from gynecologic oncologists was associated with higher Medicare spending for short stay, long stay, and skilled nursing facilities ($52 154 vs $44 203; p=0.010) compared with care from medical oncologists. Care from medical oncologists was associated with higher Medicare spending for physician/suppliers ($2643 vs $2350; p<0.001) and home health agencies ($1262 vs $1107; p=0.023) compared with care from gynecologic oncologists. There was no significant difference in Medicare spending for the following claim types by primary oncologist specialty: institutional outpatient providers (p=0.482), hospice (p=0.158), durable medical equipment (p=0.116), and Part D (p=0.475).
Difference in Medicare spending in the last 30 days of life by primary outpatient oncologist type
Discussion
Summary of Main Results
Compared with patients who receive the majority of their cancer care from a medical oncologist, those who receive the majority of their cancer care from a gynecologic oncologist are less likely to receive high-intensity care, but more likely to undergo invasive procedures at the end of life. Overall, both specialties engage in high levels of intense end-of-life care, with rates consistent with those previously reported.22–24 Similar to prior literature,16 most patients who experienced high-intensity end-of-life care experienced only one measure of aggressive care. In addition, we found that patients with a primary gynecologic oncologist have higher Medicare spending in the last month of life compared with patients with a primary medical oncologist. Overall, these findings indicate that gynecologic oncology patients receive a high amount of aggressive medical end-of-life care, which may have implications for their quality of life at the end of life. Given the differences in end-of-life care between patients with a primary gynecologic oncologist versus a medical oncologist, physician-level training could be a target for educational or quality improvement initiatives to improve end-of-life cancer care delivery.
Results in the Context of Published Literature
We found higher rates of invasive procedures at the end-of-life than what has previously been reported. Lower rates of invasive procedures in other literature may be due to variation in inclusion criteria, as there is no validated standard definition.7 23 Silber et al found no differences in operative procedures in the 5 years after the initial staging procedure by oncologist specialty.7 Our finding that patients with a primary gynecologic oncologist are more likely to undergo invasive procedures in the last month of life may indicate that the timing of procedures over a patient’s cancer course differs by primary oncologist specialty, and that gynecologic oncologists are more likely to offer invasive procedures, even in patients with a very poor prognosis. Many of these invasive procedures may be performed with palliative intent, but we were not able to determine the intent of the procedure in our data. Furthermore, our analysis assumed that the primary oncologist would be determining the appropriateness of a procedure, and we did not assess the specialty of the provider directly performing the procedure.
Differences in Medicare spending at the end of life by oncology specialty have not been previously reported. It has been established that cancer care spending increases at the end-of-life, driven mainly by inpatient spending.25 26 Similarly, the majority of our observed differences in Medicare spending at the end of life are due to differences in inpatient spending, with gynecologic oncologist patients having significantly higher Medicare spending. While we do not find specialty differences in hospitalizations or ICU admissions in the last month of life to explain spending differences, other studies have suggested that gynecologic cancer patients with high-cost admissions were more likely to undergo invasive procedures.27 Therefore, it is possible that the higher rate of invasive procedures among gynecologic oncologist patients are contributing to higher Medicare spending.
This study captures new dimensions of specialty differences in cancer care compared with descriptions in previous work. A previous study that examined the role of the oncologist specialty in gynecologic cancer care was conducted by Silber et al (2007), who examined differences in care provided in the first 5 years of treatment. While the study found no differences in survival, it did find that gynecologic oncologist patients received less chemotherapy with fewer weeks of chemotherapy-associated toxicity than medical oncologist patients.7 Our findings suggest that specialty differences in chemotherapy treatment may persist throughout a patient’s cancer course, with medical oncologists being more likely to prescribe chemotherapy, even in the last weeks of life.
In a recently published study, Mullins et al (2021) found that physician characteristics did influence end-of-life care, although physician specialty was not meaningfully associated with variations in end-of-life care.8 Similar to our study, they also used SEER-M data and found a similar distribution of patients to gynecologic oncologists versus medical oncologists at the end of life. However, they defined physician specialty differently, assigning physicians to gynecologic oncology, OB/GYN, oncology, or other. Their analyses also did not compare outcomes among patients with gynecologic oncologists versus those with medical oncologists directly. Furthermore, their study did not evaluate healthcare spending outcomes.
Strengths and Weaknesses
Our study provides a unique perspective on how oncologist specialty may be associated with differences in treatment course even in the last weeks of life. Strengths of the study include our large cohort from a national sample. Our careful definitions of the medical specialties allowed us to directly compare patients of only medical versus gynecologic oncologists. This comparison more accurately reflects how gynecologic cancer care is delivered in the United States compared with other literature. Our analyses by Medicare claim type provide a detailed examination of Medicare spending at the end of life. Finally, we performed several sensitivity analyses to evaluate the robustness of our findings.
Our study has several limitations. First, our cohort was limited to older patients who aged into Medicare and were enrolled in both Parts A and B. Practice patterns may be different for younger patients or those with commercial insurance or Medicare Advantage, which is an alternate insurance plan where Medicare services are subcontracted and overseen by commercial (private) insurers. Studies have found that end-of-life spending25 and palliative care specialist care28 are higher for younger patients and that patients with Medicare Advantage tend to use hospice more and hospital services less at the end of life compared with patients with fee-for-service Medicare.29 30 However, there are data that beneficiaries enrolled in Medicare Advantage do not differ significantly in characteristics compared with those in traditional Medicare.31 Second, it is possible that we misclassified a physician’s specialty because the specialty codes could be incorrect. In addition, it is not uncommon in gynecologic oncology for patients to receive care from both gynecologic and medical oncologists in a team-based collaborative care model. We therefore could also have misclassified primary oncologist because the assignment was based on a plurality of visits, assuming that the physician who sees a patient most frequently is the most involved with clinical decision-making. We would expect measurement errors like these to bias our results toward the null. Furthermore, our results remained consistent in our sensitivity analyses. Third, high-intensity end-of-life care may represent goal-concordant care for an individual patient. We were unable to assess goal-concordance in our data. Fourth, while clinical practice may change over time, we did not find changes in aggressive end-of-life care over time in our prior work.32 Finally, we were not able to accurately assess palliative care referrals or consultations.
Implications for Practice and Future Research
This is the first study to examine differences in end-of-life care by primary oncologist specialty. Overall, the majority of gynecologic cancer patients experience high-intensity end-of-life care. Differences in high-intensity end-of-life care, invasive procedures, and Medicare spending by primary oncologist specialty indicate that training background and scope of practice have a measurable impact on the care that patients receive. Interventions to address high rates of high-intensity end-of-life care and differences by primary oncologist specialty may include additional training,33 support for earlier end-of-life discussions,34 increased and more consistent referral to palliative care specialists,15 35 and clear documentation of advance directives,36 allowing patients to make deliberate decisions about their cancer care at the end of life.37
Conclusions
End-of-life care with a gynecologic versus medical oncologist was associated with lower rates of high-intensity end-of-life care, higher rates of invasive procedures, and higher Medicare spending in the last month of life.
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Acknowledgments
We thank Dr. Lilie Lin for her contributions in funding support and assistance in development of prior Surveillance, Epidemiology and End Results-Medicare (SEER-M) analytic datasets which further supported the conduct of this current project.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @khickscourant
Presented at Preliminary results of this study have been presented at the Mid-Atlantic Gynecologic Oncology Society Annual Meeting (October 2020), the Society of Gynecologic Oncology Annual Meeting (March 2021), and the American Society of Clinical Oncology Annual Meeting (June 2021).
Contributors KH-C: conceptualization, methodology, software, formal analysis, writing – original draft, writing – review and editing, guarantor. GPK: methodology, formal analysis, writing – review and editing, supervision. MMS: conceptualization, methodology, writing – review and editing, supervision. CMB: software, data curation, writing – review and editing. QL: software, data curation, writing – review and editing. EMK: conceptualization, methodology, data curation, project administration, funding acquisition, writing – review and editing, supervision.
Funding This study was supported in part by grants: Leonard Davis Institute 2019 Pilot Grant Program (PI: EMK); American Cancer Society 124268-IRG-78-002-35-IRG (PI: EMK); by the George and Emily McMichael Harrison Fund, Penn Presbyterian Harrison Fund of the University of Pennsylvania Hospital Obstetrics and Gynecology Department (PI: EMK); and a donation in-kind to the University of Pennsylvania Department of Radiation Oncology (PI: Lilie Lin). KHC acts as the guarantor.
Competing interests EMK reports grants from Tesaro, outside the submitted work.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.