Article Text
Abstract
Objectives The role of adjuvant treatment for early-stage uterine serous carcinoma is not defined. The goal of this study was to investigate the impact of adjuvant treatment on survival of patients with tumors confined to the endometrium.
Methods Patients diagnosed with stage I uterine serous carcinoma with no myometrial invasion between January 2004 and December 2015 who underwent hysterectomy with at least 10 lymph nodes removed were identified from the National Cancer Database. Adjuvant treatment patterns defined as receipt of chemotherapy and/or radiotherapy within 6 months from surgery were investigated and overall survival was evaluated using Kaplan–Meier curves, and compared with the log-rank test for patients with at least one month of follow-up. A Cox analysis was performed to control for confounders.
Results A total of 1709 patients were identified; 833 (48.7%) did not receive adjuvant treatment, 348 (20.4%) received both chemotherapy and radiotherapy, 353 (20.7%) received chemotherapy only, and 175 (10.2%) received radiotherapy only. Five-year overall survival rates for patients who did not receive adjuvant treatment (n=736) was 81.9%, compared with 91.3% for those who had chemoradiation (n=293), 85.1% for those who received radiotherapy only (n=143), and 91.0% for those who received chemotherapy only (n=298) (p<0.001). After controlling for age, insurance status, type of treatment facility, tumor size, co-morbidities, and history of another tumor, patients who received adjuvant chemotherapy (HR 0.64, 95% CI 0.42, 0.96), or chemoradiation (HR 0.55, 95% CI 0.35, 0.88) had better survival compared with those who did not receive any adjuvant treatment, while there was no benefit from radiotherapy alone (HR 0.85, 95% CI 0.53, 1.37). There was no survival difference between chemoradiation and chemotherapy only (HR 1.15, 95% CI 0.65, 2.01).
Conclusion Adjuvant chemotherapy (with or without radiotherapy) is associated with a survival benefit for uterine serous carcinoma confined to the endometrium.
- uterine cancer
- radiation
Statistics from Altmetric.com
HIGHLIGHTS
Among 1709 patients with uterine serous carcinoma confined to the endometrium, 48.7% did not receive adjuvant treatment.
Adjuvant chemotherapy and chemoradiation were associated with better survival compared with observation.
There was no difference in survival between observation and radiation only.
Introduction
Endometrial cancer is the most common gynecologic malignancy in the United States.1 Uterine serous carcinoma accounts for approximately 10% of all malignant endometrial tumors but is responsible for 39% of uterine cancer-related deaths.2 Uterine serous carcinoma is associated with a distinct molecular profile, exhibits aggressive features, and a propensity for distant metastases.2 3 Given the high rate of relapse, the optimal adjuvant treatment regimen for patients with disease confined to the endometrium is currently a subject of debate.2 The use of platinum-based chemotherapy has been associated with a survival benefit for patients with early-stage disease and is commonly offered.4 Given the low incidence of uterine serous carcinoma, to date there is no phase III trial investigating the role of adjuvant treatment exclusively for patients with this histology. Serous histology is usually grouped along with other histotypes, precluding the safe generalization of trial outcomes.5
According to the current National Comprehensive Cancer Network (NCCN), for patients with stage IA disease observation (for patients with no residual disease in the hysterectomy specimen), chemotherapy with or without vaginal brachytherapy, and external beam radiation therapy with or without vaginal brachytherapy are all appropriate management options.6 However, recommendations are not modified by the presence or absence of myometrial invasion. For patients with tumors without myometrial invasion (confined to the endometrium) the decision to administer adjuvant treatment must balance the aggressive nature of uterine serous carcinoma and the long-term therapy-related toxicities that can affect the patient’s quality of life. Several retrospective studies have investigated the role of adjuvant treatment for these patients but are limited due to the small number of patients included and the lack of adequate statistical power, reaching conflicting results.7–12 The aim of the present study was to evaluate the impact of adjuvant treatment on the survival of surgically staged patients with uterine serous carcinoma without myometrial invasion using a large multi-institutional database.
Methods
A cohort of patients diagnosed between January 2004 and December 2015 with a malignant tumor of the uterus was selected from the National Cancer Database (NCDB), a hospital-based database capturing approximately 70% of all malignancies diagnosed in the United States. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytical or statistical methodology employed, or the conclusions drawn from these data. The present study was deemed exempt from Institutional Board Review from Penn Medicine (Protocol #829268).
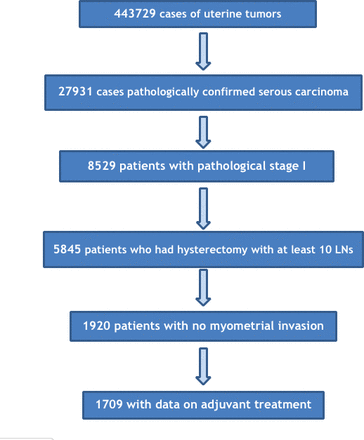
Using histology codes (8441, 8460, 8461), patients with uterine serous carcinoma with pathological stage I disease, who had a hysterectomy were identified. Based on the collaborative staging schema extent of disease variable and pathology report, patients with disease confined to the endometrium (no myometrial invasion) with no lymph node or distant metastasis were selected for further analysis. Given the relatively high prevalence of lymph node metastases we opted to include only patients who had lymphadenectomy with at least 10 regional lymph nodes removed as indicated by the pathology report. Receipt of adjuvant chemotherapy and radiation therapy (vaginal brachytherapy and/or external beam radiation) was defined as treatment received within 6 months from definitive surgery. Patients who received treatment before surgery, those with unknown interval, or patients who received treatment more than 6 months from surgery were excluded. Figure 1 depicts the patient selection flowchart.
Patient selection flowchart. LN, lymph node.
Demographic, clinicopathological, and treatment variables were extracted from the de-identified dataset. For analysis purposes, patient race was recoded into White, Black, and Other/Unknown and insurance status into Private, Government (including Medicaid and Medicare), and Uninsured/Unknown. Based on prior studies, age was grouped into <65 and ≥65 years to define an older population.13 14 The presence of co-morbidities was assessed from the Charlson–Deyo Comorbidity Index, a clinical co-morbidity index designed for use with medical records that takes into account the severity of co-morbid conditions and classified as absent (score 0) or present (score ≥1).
The frequency of distribution of categorical variables was compared with the Chi-square test or Fisher’s exact test and continuous variables with the Kruskall–Wallis test. Overall survival was assessed for patients diagnosed between 2004 and 2014 who had at least 1 month of follow-up. Kaplan–Meier curves were generated to determine 5-year overall survival rates, while univariate analysis was performed with the log-rank test. In the NCDB, overall survival is reported as months from diagnosis to last follow-up or death. Variables associated with survival by univariate analysis were identified and entered into a Cox multivariate model. In a sensitivity analysis, the impact of chemotherapy and radiation therapy was evaluated among patients without a history of another tumor or co-morbities, as well as among those with known tumor size. All statistical analyses were performed with the Statistical Package for the Social Sciences v.24 statistical package (IBM Corporation, Armonk, NY, USA) and the alpha level of statistical significance was set at 0.05.
Results
A total of 1709 patients who met the inclusion criteria were identified. Median patient age was 66 (range 53–90) years, while the majority were White (73%), with government-issued insurance (54.1%), without any co-morbidities (76.9%), or a history of another tumor (79.6%), and were managed in academic facilities (55%). The median number of regional lymph nodes removed was 19 (range 10–81). Para-aortic sampling/dissection data were available for 1132 patients, and of those 76.6% had a para-aortic lymphadenectomy performed. Data on the performance of omentectomy was available for 1014 patients; of those, 54% had omentectomy. Status of peritoneal cytology was available for 882 cases; of those, 6.8% (60 cases) had positive washings. Moreover, based on data from 983 cases, the presence of lymphovascular invasion was rare (2.5%, 42 cases).
Almost half (48.7%) of the patients did not receive adjuvant treatment. A total of 353 patients (20.7%) received adjuvant chemotherapy only. The combination of chemotherapy and radiation therapy was used in 348 (20.4%) patients (86.2% vaginal brachytherapy, 7.2% external beam radiation, 6% combination of both, and 0.6% unknown type of radiation). Radiation therapy only was administered in 175 patients (10.2%) (83.5% vaginal brachytherapy, 9.7% external beam radiation, 5.7% combination of both, and 1.1% unknown type of radiation). Median interval from surgery to chemotherapy and radiation therapy administration was 41 and 72 days, respectively. Demographic and clinicopathological characteristics of patients stratified by type of adjuvant treatment are presented in Table 1. Online supplementary table 1 compares the demographic and clinicopathological characteristics of patients who did and did not receive adjuvant treatment.
Supplemental material
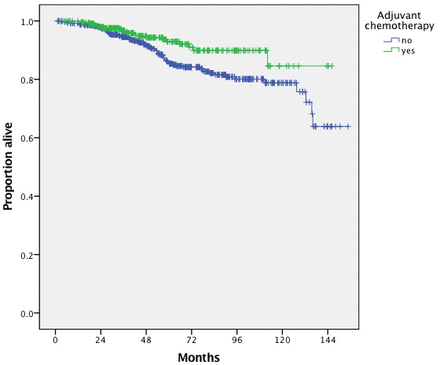
Median follow-up of the entire cohort was 58.55 months; 64.89 months in the observation group, 49.15 months in the chemotherapy only group, 58.61 months in the radiotherapy only group, and 51.75 months in the chemoradiation group, respectively. Online supplementary table 2 summarizes factors associated with overall survival by univariate analysis. Patients who received chemotherapy (with or without radiotherapy) (n=591, 50 deaths) had better overall survival compared with those who did not (n=879, 154 deaths) (p<0.001); 5-year overall survival rates were 91.2% (95% CI 88.2%, 94.2%) and 82.4% (95% CI 79.4%, 85.4%), respectively. Patients who received radiotherapy (n=436, 42 deaths) had better overall survival compared with those who did not (n=1034, 162 deaths) (p=0.015); 5-year overall survival were 89.1% (95% CI 85.5%, 92.7%) and 84.2% (95% CI 81.4%, 87%). Five-year overall survival rates for patients who did not receive any adjuvant treatment (n=736, 134 deaths) was 81.9% (95% CI 78.5%, 85.3%), compared with 91.3% (95% CI 87.2%, 95.4%) for those who had chemoradiation (n=293, 22 deaths), 91% (95% CI 86.9%, 95.1%) for those who received chemotherapy only (n=298, 28 deaths), and 85.1% (95% CI 78%, 92.2%) for those who received radiotherapy only (n=143, 20 deaths) (p<0.001) (Figure 2).
Supplemental material
After controlling for patient age (<65, >=65 years), insurance status (private, government-issued), type of treatment facility (academic, non-academic), tumor size (<2 cm, >=2 cm, unknown), presence of co-morbidities, and history of another tumor, patients who received adjuvant chemotherapy (HR 0.64, 95% CI 0.42, 0.96) and patients who received chemoradiation (HR 0.55, 95% CI 0.35, 0.88) had better survival compared with those who did not receive any adjuvant treatment, while there was no benefit from radiotherapy alone (HR 0.85, 95% CI 0.53, 1.37) (Table 2). There was no survival difference between patients who received chemoradiation and chemotherapy only (HR 1.15, 95% CI 0.65, 2.01). When entering into the Cox model adjuvant chemotherapy and radiation therapy as separate variables along with their interaction, only chemotherapy (HR 0.64, 95% CI 0.42, 0.96) and not radiation therapy (HR 0.85, 95% CI 0.53, 1.37) was associated with better survival.
In a sensitivity analysis we evaluated the impact of chemotherapy use on the survival of patients who did not have co-morbidities and/or a history of another tumor. Median follow-up in the observation and chemotherapy groups were 63.8 and 49.2 months, respectively. Patients who received adjuvant chemotherapy (n=355, 21 deaths) had better overall survival compared with those who did not (n=516, 69 deaths) (p=0.012); 5-year overall survival rates were 92.9% (95% CI 89.5%, 96.3%) and 85.3% (95% CI 81.5%, 89.1%), respectively (Figure 3). However, patients who received radiotherapy (n=273, 22 deaths) did not have better survival compared with those who did not (n=598, 68 deaths) (p=0.25); 5-year overall survival rates were 90.2% (95% CI 85.7%, 94.8%) and 87.1% (95% CI 83.7%, 90.5%), respectively. After controlling for other confounders, use of adjuvant chemotherapy was associated with better survival (HR 0.59, 95% CI 0.36, 0.97). When limiting our analysis to patients with known tumor size, again use of adjuvant chemotherapy was associated with better survival after controlling for confounders (HR 0.64, 95% CI 0.42, 0.96).
Clinicopathological characteristics of patients with International Federation of Gynecology and Obstetrics (FIGO) stage I uterine serous carcinoma confined to the endometrium stratified by type of adjuvant treatment
Overall survival of patients with International Federation of Gynecology and Obstetrics (FIGO) stage I uterine serous carcinoma confined to the endometrium stratified by type of adjuvant treatment. CT, chemotherapy; RT, radiation therapy.
Overall survival of patients with International Federation of Gynecology and Obstetrics (FIGO) stage I uterine serous carcinoma confined to the endometrium without a history of another tumor or co-morbidities stratified by receipt of adjuvant chemotherapy.
Results of multivariate survival analysis.
Discussion
This is the largest cohort of patients with surgically staged uterine serous carcinoma with no myometrial invasion in which the effect of adjuvant treatment on overall survival has been evaluated. Almost half of the patients did not receive adjuvant treatment and had a worse survival compared with those who did. We further demonstrated that the administration of adjuvant chemotherapy may be associated with a survival benefit in this patient population, while the impact of radiation therapy alone was not significant.
Given the relative low burden of disease in patients with uterine serous carcinoma confined to the endometrium and the significant side effects of radiation therapy and chemotherapy, several retrospective studies have investigated the role of adjuvant treatment in this patient population in an attempt to identify subsets that can be spared from adjuvant treatment. Several authors quote a low relapse rate for adequately staged patients and suggest that observation alone is a reasonable option. Havrilesky et al examined the outcomes of 83 patients with stage I uterine serous carcinoma and patients with disease limited to the endometrium had excellent outcomes without adjuvant treatment (n=22, 5% relapse rate).8 In another cohort of 21 patients with uterine serous carcinoma confined to the endometrium, 70% received adjuvant treatment, and only one recurrence was observed.15 No recurrences were detected among 15 patients (93% had residual tumor in the hysterectomy specimen) with stage IA uterine serous carcinoma irrespective of post-operative therapy in a study from the Mayo Clinic.7
In a retrospective study from Memorial Sloan Kettering that included 85 surgically staged patients with high-grade tumors (56 with pure serous histology) and no myometrial invasion, a total of five relapses were noted and the majority (80%) were observed in patients who had received adjuvant treatment.16 The authors concluded that surveillance alone appears to be an appropriate management approach for adequately staged patients with stage IA high-grade tumors with disease limited to a polyp or the endometrium in the absence of myometrial invasion.16 In an analysis of the British Columbia tumor registry that included 41 patients with uterine serous carcinoma limited to the endometrium (six with no residual disease), the majority (30 patients, 73.2%) did not receive adjuvant treatment.11 Relapse rate was 12.2% (5/41) and was comparable between the observation (10%, 3/30) and adjuvant treatment groups (2/11, 18.2%).11 Lack of survival benefit may be related to the small number of patients included and the absence of statistical power.
Similar to our study, a relatively high relapse rate was reported by Mahdavi et al (2001) in a group of patients with serous carcinoma confined to the endometrium who did not receive any adjuvant chemotherapy (30%, 3/10).9 In a multi-institutional study from nine academic centers of patients with serous carcinoma who had comprehensive surgery, relapse rates for patients with tumors confined to the endometrium were 15.8% (3/19) in the observation group, compared with 7.4% (2/27) in the chemotherapy±radiotherapy and 20% (1/5) in the radiotherapy only groups (p=0.54).4
The presence and size of residual disease in the hysterectomy specimen may influence the risk of tumor relapse. In a study by Kelly et al, among 12 patients with serous carcinoma confined to the endometrium who did not have residual tumor in the hysterectomy specimen after a median of 38 months there were no recurrences regardless if patients did (n=3) or did not (n=9) receive adjuvant treatment. However, among patients with residual disease, the relapse rate was 43% among those who did not receive chemotherapy (n=14) compared with 0% for those who did (n=7).10 In a recent study that included 26 patients with high-grade endometrial cancer and minimal or no invasive disease on final pathology specimen, adjuvant treatment was given to 12 patients with no impact on the risk of recurrence.4 Currently the NCCN guidelines suggest that for patients with stage IA disease observation can be offered to selected patients with uterine serous carcinoma and no residual tumor in the hysterectomy specimen. For all other patients, chemotherapy with or without vaginal brachytherapy and external beam radiation therapy with or without vaginal brachytherapy are all acceptable management options. Based on the same guidelines, administration of a doublet therapy such as carboplatin/paclitaxel is preferred if tolerated.
In our study, radiation therapy was not associated with a statistically significant survival benefit. The use of external beam radiotherapy was rare in this patient population. Among the 523 patients who received radiation therapy, only 73 patients received external beam radiotherapy, 31 of whom in combination with vaginal brachytherapy. Similarly, in a prospective study that included 21 patients with clinical stage I-II uterine serous carcinoma treated with radiotherapy, 8/19 patients died of recurrent disease, five of whom developed a recurrence within the radiation field.17 In another retrospective study, the risk of relapse and survival were comparable between patients with surgical stage I disease who received adjuvant radiotherapy without chemotherapy (n=12) and those who did not receive any adjuvant treatment (n=40) (17 vs 16%).18 Whether patients without myometrial invasion can be spared radiation therapy and be managed with chemotherapy alone should be further investigated.
Certain limitations of the present study should be noted. While all patients had disease limited to the endometrium, the exact size of the residual tumor on the hysterectomy specimen was not available for approximately one-third of cases. Moreover, given the absence of a central pathology review and data on the quality of the staging procedure performed, possible tumor and staging misclassifications cannot be excluded. We could not assess whether patients who did not undergo omentectomy had omental biopsies instead. By including only patients who had at least 10 lymph nodes removed we may have inadvertently excluded a small number of adequately staged patients who underwent sentinel lymph node biopsy. Due to the lack of information on cause of death and tumor relapse, analysis of cancer-specific and progression-free survival was not feasible. Also, information on the chemotherapy regimens (agents, dosage, and number of cycles) used in the management of each patient was not available. Unmeasured factors influencing clinician’s decision to administer adjuvant chemotherapy such as patient’s functional status or primary physician’s subspecialty were not available, while median follow-up was longer in the observation only group. Lastly, for analysis purposes we combined all types of radiation and could not investigate the impact of each modality separately given the low number of patients.
In this large cohort of surgically staged patients with stage I uterine serous carcinoma confined to the endometrium the administration of adjuvant chemotherapy (with or without radiation therapy) was associated with a survival benefit. However, in the absence of level 1 data, decisions should be individualized following extensive counseling. The results of the present study should be regarded as hypothesis generating. Large prospective studies with international participation that would include patients with limited tumor burden (confined to a polyp or endometrium) could potentially elucidate the optimal management of these patients.
Visual abstract
References
Footnotes
Contributors All authors contributed significantly to the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data may be obtained from a third party and are not publicly available. Data from the National Cancer Database.