Article Text
Abstract
Objectives The aim of this study was to evaluate the impact of micrometastasis and isolated tumor cells on disease recurrence in patients with early-stage cervical cancer.
Methods We included patients with International Federation of Gynecology and Obstetrics (FIGO) stage IA1 with lymphvascular space invasion, stage IA2, and IB1 who participated in the SENTICOL1 trial. A centralized histologic analysis with re-reading and ultrastaging was performed 3 months after surgery and treatment was not impacted by findings from our study. Patients were followed for 3 years and outcomes were compared according to prognostic factors.
Results A total of 139 patients were included and 13 recurrences were found. There were two recurrences in patients with positive sentinel lymph node (SLN) (one macrometastases and one micrometastases) and 11 recurrences in patients with negative lymph nodes (sentinel or non-sentinel). Among patients with positive SLN for micrometastases there was only one recurrence. No patient with isolated tumor cells on their lymph nodes experienced a recurrence. There was a significant decrease in disease-free survival in patients aged >50 years (p = 0.01).
Conclusion Evidence of micrometastasis or isolated tumor cells in the SLN of untreated patients with early cervical cancer in the SENTICOL1 trial did not impact progression-free survival.
- cervical cancer
- sentinel lymph node
- micrometastasis
Statistics from Altmetric.com
HIGHLIGHTS
In early-stage cervical cancer, micrometastasis in sentinel lymph node (SLN) seems to have no impact on disease-free survival.
No recurrence occurred in any of the patients with SLN positive for isolated tumor cells.
Patient age shows a statistically significant difference in disease-free survival.
Introduction
Lymph node status is the most important prognostic factor for survival in women with early-stage cervical cancer. In a recent review,1 lymph node involvement in early-stage cervical cancer is estimated to be approximately 15%–20% and it varies according to stage of disease,2 3 which suggests that the majority of early cervical cancer patients undergoing pelvic lymph node dissection are exposed to unneccessary morbidity.4 Sentinel lymph node (SLN) biopsy is a safe and effective strategy for detection of lymph node metastases and for reducing the incidence of lower extremity lymphedema.5 Use of the SLN technique in women with cervical cancer can reduce morbidity, seemingly without altering disease-free survival.4–6 In the 2015 NCCN Guidelines, SLN mapping was considered an alternative to lymphadenectomy (category 2B).7
In patients with early-stage cervical cancer, SLN biopsy is purported to be a sensitive method for detecting lymph node involvement, especially if the SLN is detected bilaterally,1 8 with a high sensitivity and a negative predictive value of 99%.9 According to a review by Tax ,10 the false-negative rate is considered very low, with a residual risk of occult metastases of 0.08% in non-sentinel lymph nodes in cases of no suspicious lymph nodes on pre-operative imaging or during surgery, and bilateral negative SLN after ultrastaging. In fact, with pathologic ultrastaging, one may achieve a sensitivity of 100% for the presence of macrometastasis and micrometastasis in pelvic lymph nodes.11
Although pathologic ultrastaging of SLN allows for identification of low-volume disease in up to 15% of cases, including micrometastases and isolated tumor cells, the prognostic significance of these findings is unknown.12
In retrospective studies, presence of micrometastasis is an important risk factor for tumor recurrence in patients with early cervical cancer.12 13 In a study by Cibula et al, presence of micrometastases in the SLN was associated with significant reduction of overall survival, which was equivalent to patients with macrometastasis. However, no prognostic significance was found for isolated tumor cells.12 A retrospective multicenter study14 showed that patient survival was not altered by lymphadenectomy in case of positive or negative SLN, but was significantly improved (p=0.046) by lymphadenectomy (>16 lymph nodes) when SLN was positive for micrometastasis or isolated tumor cells. However, it should be noted that this result is only observed for patients with tumors of stage IB2 and above. For patients with tumors of stage IA or IB1, the number of lymph nodes removed during lymphadenectomy has no impact on patient survival.
The SENTICOL1 trial was designed to assess the diagnostic accuracy of a standardised SLN biopsy technique in patients with early cervical cancer. The primary objective of this study was to assess the sensitivity and negative predictive value of the SLN biopsy by using histologic examination of a full lymphadenectomy specimen as the reference standard.8 One of the secondary objectives of the SENTICOL1 trial was to assess the 3-year disease-free survival of patients and to define the impact on recurrence rates of the presence of micrometastasis and isolated tumor cells. This secondary objective is the primary aim of this current study.
Methods
Patient Selection
We conducted a prospective longitudinal study in seven centres in France between January 2005 and June 2007. The SENTICOL1 trial was approved by the Comité de Protection des Personnes HEGP-Broussais. Consecutive patients were enrolled prospectively and followed for 3 years.8
We included patients with cervical carcinoma with International Federation of Gynecology and Obstetrics (FIGO) stage IA1 (lymph-vascular space invasion) to IB1, and histologic subtypes such as squamous, adenocarcinoma, or adenosquamous carcinoma. Written informed consent was obtained from all patients.
The radioactive tracer colloidal rhenium sulfide labeled with technetium [99mTc] (Nanocis, 120MBq, Cis Bio International, Gif sur Yvette, France) and then 2.5% Patent Blue (2 mL diluted in 2 mL saline; Bleu Patenté V sodique, Guerbet, Roissy, France) were injected into the cervix at the 3, 6, 9, and 12 o’clock positions. After laparoscopic identification of the SLN, all patients underwent pelvic lymphadenectomy. Intra-operative frozen sections were performed on the SLN in 30 (21.6%) patients. If the intra-operative frozen section result was positive for nodal metastasis, patients underwent pelvic and para-aortic lymphadenectomy, radical surgery, and adjuvant radio-chemotherapy. If the intra-operative frozen section was negative, pelvic lymphadenectomy and radical surgery were performed. In one centre, the treatment protocol included pre-operative brachytherapy for tumors larger than 2 cm in diameter: 22 (16%) patients underwent this treatment.
After surgery, all lymph nodes underwent standard histologic examination: nodes were sectioned every 2 mm and stained with hematoxylin-eosin-saffron. At that time, adjuvant treatment was performed based on the presence of these criteria: positive nodes or vaginal/parametrial invasion, or tumor larger than 4 cm. In addition, three patients with multiple positive lymph-vascular invasion underwent adjuvant treatment. Adjuvant treatment consisted of chemotherapy and radiation with treatment fields adapted to the extension of the lymph node involvement.
A re-reading of the histologic sections and lymph node ultrastaging was performed 3 months after the original surgery in two centralized histologic centres on all lymph nodes (sentinel and non-sentinel). The reason for performing ultrastaging on all nodes was to be certain that the negative predictive value of the SLN technique was adequate and that micrometastasis or isolated tumor cells in the non-sentinel nodes were not missed. All nodes were sectioned every 200 µm and stained with hematoxylin-eosin-saffron. In cases of negative hematoxylin-eosin-saffron staining, a section from the same level was examined using immunohistochemistry with the pan-cytokeratin antibody AE1-AE3 (DAKO, Trappes, France). Isolated tumor cells were defined as cells or masses of cells measuring ≤0.2 mm, micrometastasis as a tumor >0.2 mm and ≤2 mm, and macrometastasis as a tumor >2 mm. Discovery of micrometastasis or isolated tumor cells in secondary ultrastaging analysis did not alter the adjuvant treatment as these data were obtained more than 3 months after the surgical treatment.
Data Collection
All data were prospectively entered into a database, and the 3-year follow-up was defined as one of the secondary objectives of the SENTICOL1 trial. Standard, guideline-based, clinical follow-up was performed (every 3–4 months with clinical examination and annual Pap-smear test) for a minimum of 3 years. Disease-free survival was evaluated with respect to nodal status, histologic type, tumor diameter, age, presence of lymph-vascular invasion, and pre-operative brachytherapy treatment.
Statistical Methods
Standard summary statistics were used and Chi–square test was applied to assess categorical data. A value of p=0.05 was used as the limit of statistical significance in all other parametric analyses. Kaplan–Meier method was used to describe the relapse-free survival. Relapse-free survival was calculated as the time interval between time of diagnosis and time when disease recurrence was identified. Log-rank test was applied to compare survival in different groups of patients in stratified survival analyses. Both univariable and multivariable proportional hazard Cox regression models were applied to quantify association of potential risk factors and survival endpoints. Wald Chi-square test was performed to estimate the HR (supplied with 95% CIs).
Results
A total of 139 patients were included in the SENTICOL1 trial.8 Concerning type of radical surgery, a radical hysterectomy was performed in 88 (63%) patients, a modified radical hysterectomy was performed in the 22 (16%) patients treated with pre-operative brachytherapy, a vaginal radical trachelectomy (fertility-sparing surgery) was performed in 27 (19%) patients, and two patients refused any uterine surgery. Of note, all patients underwent identification of the SLN by laparoscopy; however, 14/139 patients (10%) underwent radical hysterectomy by laparotomy and the remaining 125 patients underwent laparoscopic radical hysterectomy.
Histologic Results
We detected the SLN (unilateral or bilateral) in 136/139 patients (98%). The three patients with no SLN detected on either side of the pelvis and the 28 patients with an unilateral SLN detection were included in the follow-up analysis. In total 454 SLN were analyzed. After pelvic lymphadenectomy, 2056 non-SLN were identified with a median number per patient of 13 (range 1–54) lymph nodes.
In eight patients, a macrometastases was detected in a minimum of one SLN (five patients with one unilateral macrometastases, one patient with macrometastases and contralateral isolated tumor cells after ultrastaging, one patient with two ipsilateral macrometastases, and one patient with bilateral macrometastases). All macrometastases were identified in standard analysis. After ultrastaging, in 13 patients eight SLN were positive for micrometastases and eight for isolated tumor cells (including one patient with micrometastasis and isolated tumor cells on the same side, one patient with bilateral micrometastases and isolated tumor cells on the left side, one patient with micrometastases on one side and isolated tumor cells on the other side). Ultrastaging detected additional metastases in 10% of patients (eight micrometastases and eight isolated tumor cells) compared with the standard pathological analysis. In total, 26 SLN were positive in 21 patients (Table 1). There were a total of eight patients who had positive non-SLN, four patients with positive SLN on the same pelvic side, and four with negative or non-detected SLN.
Analysis and outcome of patients with positive sentinel lymph node
A total of 17 patients had only the SLN positive; four patients had SLN and metastasis in non-SLN; four patients had only positive non-SLNs, including one with failure of bilateral SLN detection and two with failure of the SLN detection on the pelvic side of the non-SLN metastasis. Thus, only one was a true false-negative (negative SLN in standard analysis and in ultrastaging), but one lymph node was a positive non-SLN for macrometastases. In total, 25 patients had lymph node metastases, which represented 18% of the study population. Of these 25 patients, 11 had macrometastases (42%), eight had micrometastases (33%), and six had isolated tumor cells alone (25%).
Disease-free Survival
Sixteen patients had less than a 36-month follow-up after surgery. The median follow-up was 36 (range 1–69) months. Nineteen (14%) patients received adjuvant chemoradiotherapy due to macrometastasis (11 cases, including three cases with parametrial involvement and one case with tumor >4 cm, final tumor diameter >4 cm (three cases), multiple positive lymph-vascular invasions (three cases), and parametrial involvement at final histology (two cases).
Four of 13 patients with micrometastasis or isolated tumor cells received adjuvant therapy because of other prognostic factors: one patient with positive SLN for isolated tumor cells received chemoradiotherapy treatment because of parametrial involvement. The other three patients (two with positive SLN for micrometastases and one for isolated tumor cells) received adjuvant radiotherapy because they had multiple positive lymph-vascular invasions. These four patients treated with adjuvant treatment had not had a recurrence at the time (Table 1). Finally; 4/13 (31%) patients with micrometastasis or isolated tumur cells and 9/49 (18%) patients with lymph-vascular invasion received adjuvant chemoradiotherapy.
A total of 13 patients (9%) experienced a recurrence by the 36-month median follow-up. We observed two recurrences in patients with positive SLN, one in a patient with macrometastases and one in a patient with micrometastases (Table 1). There were 11 recurrences (Table 2) in patients with negative lymph nodes (sentinel and non-sentinel). Four (31%) of the 13 patients who recurred were treated with adjuvant chemoradiotherapy.
Characteristics of patients with recurrences
The disease-free survival rate was 90.6%. We found no statistically significant difference in disease-free survival between patients with positive or negative lymph nodes: 91.6% vs 90.4%, respectively (p=0.49). (Table 3) Among patients with SLN positive for micrometastases, only one patient had a recurrence. No recurrence occurred in any of the patients with SLN positive for isolated tumor cells.
Disease-free survival and prognostic risk factor analysis
Prognostic Factors
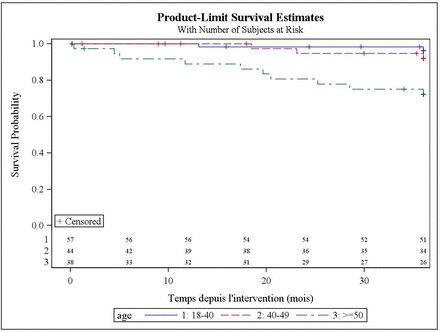
There was no statistically significant difference in disease-free survival relative to analyzed prognostic factors in early cervical cancer stages, except for age (Figure 1): the recurrence risk in patients aged ≥50 years was higher than in younger patients: 79% vs 95%, respectively (p=0.01). (Table 3)
Survival curves: disease-free survival vs patient age
Discussion
In this study, which is a follow-up of the SENTICOL1 trial, we found that evidence of micrometastasis or isolated tumor cells in the SLN of patients with early cervical cancer did not impact progression-free survival.
To date there have been no prospective studies evaluating survival rates based on volume of nodal disease in cervical cancer patients. This study is the first prospective study that analyzes as an ad-hoc analysis, the impact of micrometastasis on disease-free survival at 3 years. When comparing disease-free survival in patients having micrometastases (92.3%) there were no significant survival differences with patients with macrometastases (87.5%) or with negative lymph nodes (90.4%). We have shown that early-stage cervical cancer has a good prognosis and an acceptable survival rate of 90% after 3 years, with a low recurrence risk after treatment in our study cohort.
We had 13 (9%) recurrences in 139 patients. Lentz et al found that approximately 15% of patients with early-stage cervical cancer develop a recurrence, despite histologically negative lymph nodes.15 In our study, ultrastaging resulted in 12.4% increase in detection rate in SLN positivity. Of note, the detection of micrometastases or isolated tumor cells did not impact management and thus these patients did not receive additional therapy. Among patients with SLN positive for micrometastases, only one had a recurrence. No recurrences occurred in any of the patients with SLN positive for isolated tumor cells. This raises the question as to whether we should continue to evaluate SLN for micrometastases and isolated tumor cells with ultrastaging.
Contrary to retrospective studies,14–16 our ancillary evaluation of the SENTICOL1, as reported here, did not show any impact on disease-free survival in cases of micrometastases or isolated tumor cells. It should be noted that the SENTICOL1 study was not designed nor powered to answer the question of the impact of low-volume metastasis on oncologic outcomes. As far as other prognostic factors were concerned, only age showed a statistically significant difference in disease-free survival. Consistent with other studies,17 18 patients over the age of 50 years had an increased risk of recurrences compared with younger patients. In the cited literature, this discrepancy was explained by a more advanced disease stage in older patients who usually have less gynecologic follow-up and later discovery of the disease. It is possible that older patients may have received suboptimal surgical and/or adjuvant treatment due to age and/or co-morbidities. Our study is limited in that our cohort was too small to provide a definitive answer to the question regarding outcomes based on low-volume metastases. Another limitation of this study is the short duration of follow-up, which may have masked long-term recurrences. In addition, there were only 2 relapses in patients with positive SLN, one macrometastasis and one micrometastasis. These numbers are too low to derive definitive conclusion about the impact of low-volume metastases in the SLNs in early stage cervical cancer.
In conclusion, this is the first study evaluating prospective data from SENTICOL1 to show that neither micrometastases nor isolated tumor cells in the SLN of patients with early cervical cancer had an impact on 3-year disease-free survival. Our results question how much effort should be put into performing routine ultrastaging. Further prospective research with a larger cohort is required to ascertain whether these results are conclusive, and whether this survival rate continues beyond the 3-year time period.
Acknowledgments
The authors thank all SENTICOL study participants, especially Denis Querleu, MD PhD, Eric Leblanc, MD PhD, Philippe Morice, MD PhD, Henri Marret, MD PhD, and Emile Daraï, MD PhD. They also thank Lina Baytieh who provided assistance with English language editing, and Florent Boutitie who performed the statistical analysis.
References
Footnotes
Correction notice This paper has been amended since it was first published online. This new version has undergone a medical edit, the funding statement has been updated and the title has been changed.
Funding Part of this study (ultrastaging of non sentinel pelvic lymh-nodes) was funded by a grant from the French National Cancer Institute to P. Mathevet: INCA-PHRC 2003.
Competing interests None declared.
Provenance and peer review Not commissioned, externally peer reviewed.